

P2.9 Identifying epigenetic vulnerability in childhood blood cancers.

Project overview
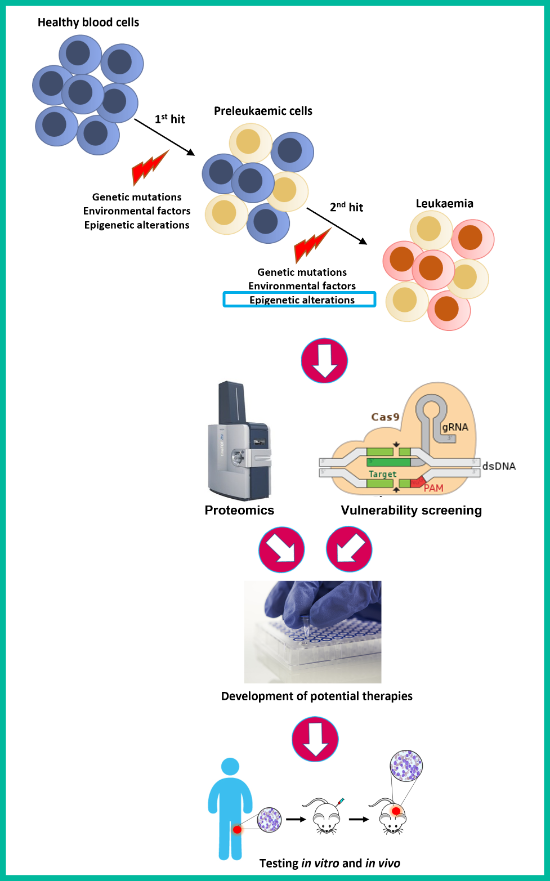
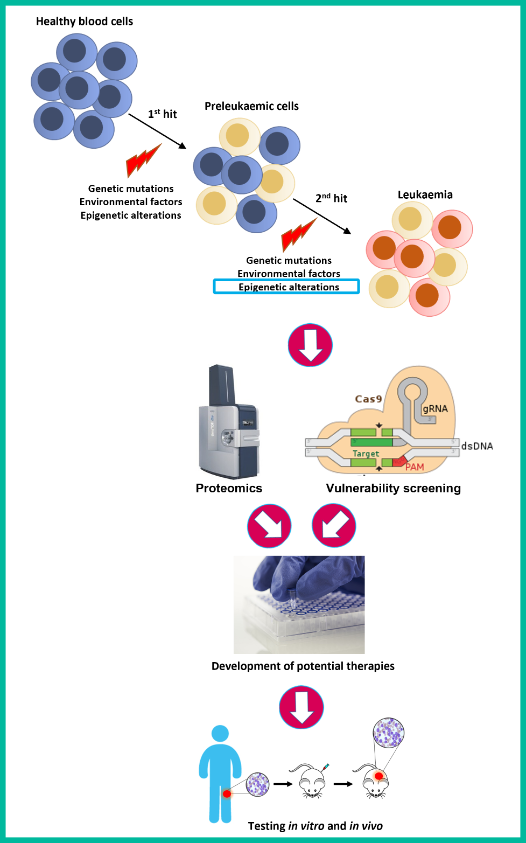
Leukaemias are the most common cancers of childhood. While outcomes for children with these diseases have improved enormously over the past few decades, much work remains to be done. Relapse rates still remain high for some poor-risk genetic subgroups, while current chemotherapy regimens can sometimes cause serious long-term side effects in children and adolescents. There is an urgent need for better and less toxic treatments that are based on more precise targeting of the molecular pathways that drive leukaemia growth and resistance.
Our research focuses on epigenetic factors, which are mutated or deleted in at least half of all human cancers. These alterations are often associated with leukaemia relapse and/or treatment resistance, suggesting that epigenetic molecules are critical modulators of blood cancer aggressiveness. There has also been growing interest in using drugs to target these factors therapeutically, and there are now a range of clinically available small molecule inhibitors that block epigenetic enzymatic activity. Excitingly, some of these agents have shown strong early promise in leukaemia, suggesting that targeting these processes is both therapeutically justified and potentially more effective than traditional non-specific chemotherapy approaches.
We have developed a collaboration between systems biology researchers at SBI and clinical colleagues at the National Children’s Cancer Service at Children’s Health Ireland at Crumlin to study the biology of epigenetic factors in high-risk childhood leukaemia. Our aim is to better understand the downstream molecular consequences of mutations and deletions in epigenetic factor genes, and to use this understanding to discover specific therapeutic vulnerabilities that will help us to identify novel precision treatments for these blood cancers.
We are working towards;
1) Assessing the proteomic and epigenomic landscape of high-risk blood cancers using cutting-edge proteomic technologies.
2) Identifying whether epigenetic alterations are associated with specific molecular vulnerabilities by performing high-throughput screening (CRISPR/Cas9 and drugs) in cancer cell lines.
3) Directly testing epigenetic treatment approaches in high-risk childhood leukaemia cells both in vitro and in vivo.
Project team


Dr. Luke Jones
Postdoctoral Researcher

