The potential of Precision Oncology
Medicine is personal, and physicians have always tried to put the patient first. More than 2,300 years ago Hippocrates, the ‘Father of Clinical Medicine’, said that “It is more important to know what sort of person has a disease than to know what sort of disease a person has.” We know that everybody is different and reacts differently to diseases and drugs. Until recently however, it has been very difficult to get a detailed enough understanding of each individual to fulfil the aspiration of treating every patient individually. But now, for the first time in history, we have technologies that allow us to distinguish the molecular differences between humans and their conditions in health and disease. We can sequence genomes, but we can also map the expression of proteins and metabolites. In combination, these molecular profiles can provide us with an understanding of risk and resistance factors inherited with our genes, but also when and why potential risks become manifest during our life, and how we are affected by our environment, lifestyle, diet and therapies we may receive – information that is better reflected in our proteins and metabolites. Putting these data together is daunting. Written out the sequence of our genome is 9 kilometres long, and the proteome and metabolome are equally complex. Thus, we need computer models to integrate, analyse and interpret these data. Using these computer models we can generate personal molecular profiles for each patient, so that we can fully understand the disease a person has and treat this individual with the best possible therapies.

Now we face a new type of challenge. Personal medicine needs personal data. Developing personal molecular profiles and personalised computer models – how will individuals and society deal with that? The benefits are clear: better therapies, less side effects, better quality of life, and more lives saved. The dangers are less tangible, but there are safeguards. Molecular information can only effectively be used for the purpose we have collected it for, and that purpose and its transparency is
regulated by strict EU and national legislation. However, we recognize that the practical interpretation can sometimes be ambiguous. This is one of many reasons why POI works with all stakeholders so that we can plot a way forward that respects personal rights, promotes research and develops new cancer therapies that are badly needed. By 2020 cancer will have surpassed cardiovascular disease as the number one killer in Ireland, and every second Irish citizen will experience cancer in their lifetime. This future is bleak. Therefore, we have to gather all the tools, all the efforts and all the stakeholders we can to put a silver lining on the horizon.
voice will be heard with the aim to radically change cancer diagnosis and treatment for patients in Ireland and worldwide.
Perspectives on setting up a Research Consortium before and during a global pandemic
I joined the Precision Oncology Ireland (POI) team at the beginning of December 2019 and immediately started helping set up the consortium in my role as Senior Research Administrator. POI consists of five Irish universities, six Irish cancer research charities, and nine companies aiming to develop new diagnostics and therapeutics for the personalised treatment of cancer. It is based out of Systems Biology Ireland at UCD, and the combined SFI, charity, and industrial funding commitment to POI will total €11.9 million over the next five years. The Consortium was officially launched on the 26th of November 2019, and since then, huge progress has been made to establish the programme. Given the complexity of POI as a unique programme which receives funding from SFI, industry partners and Irish charities you can imagine that the process of setting up the programme has not always been completely straightforward, but we are getting there. The POI operations team have been recruited, along with some members of the research team. We have negotiated the majority of our partner agreements to secure our funding. And importantly, we have engaged with academic researchers, charity and industry partners, the patient advocate community, and the public, to communicate what POI is all about.
Back in February, we raised awareness of the consortium through POI being profiled on the RTE1 ‘Nationwide’ programme, which has an almost cult following across the country and is broadcast every weekday evening, reaching audiences in every corner of Ireland. POI Director Prof. Walter Kolch and Deputy Director Prof. Liam Gallagher were both interviewed and featured in the final broadcast programme. They spoke about the research that they and their teams do and shared the importance that precision oncology and cancer research have for patients and society.

POI Director Prof. Walter Kolch walking through SBI for Nationwide segment

Photo from Nationwide segment featuring a patient involvement session in collaboration with the Patient Voice in Cancer Research
POI has also held the ‘Choirs for Cancer’ event on 4th February 2020, to mark World Cancer Day. I helped organize this event, which had over 700 attendees, 10 choirs and 300 singers was hosted by RTE presenters Miriam O’Callaghan and Marty Morrissey and brought together cancer patients, advocates, survivors, and family members to share the story of their cancer journey with the cancer research community. These stories were interspersed with inspirational songs by choirs from across the Ireland including choirs whose members were from cancer support centers or had a link to cancer. Patient advocates shared their personal cancer stories, and cancer researchers spoke of their motivation and progress towards finding new ways to prevent, diagnose and treat this devastating disease. A huge amount of planning and preparation was involved in coordinating all of the speakers and choirs. In the run up to the event I liaised with all the participating choirs who were travelling from all across Ireland to ensure they had all the information they required, helped with any logistical arrangements including parking (which was a bit of a nightmare as UCD isn’t the easiest place to secure event parking) and answered any queries they had. On the day, all of our hard-work and preparation paid off and everything ran to plan with the exception of the Sing for Life Choir’s bus breaking down an hour into their journey from Belfast to Dublin! We didn’t know if they would make it on time to perform, but luckily, they eventually got the bus back on the road and arrived in UCD halfway through the event, just in time to give a beautiful rendition of the song ‘Falling Slowly’. The event was a resounding success, with huge traction on social media (84,000 mentions of #choirsforcancer2020), and photo/video content featured on outlets including the Irish Times and the World Cancer Day highlights video. Furthermore, the event was extremely moving and heartfelt and despite the huge amount of work that went into organizing it, everyone who worked tirelessly in the background felt that the experience was definitely worth the effort and agreed that memories from the day will stay with them for years to come.
February turned out to be a very busy month for POI. We headed west to Galway to hold our first team meeting with the researchers, charity partners and industry partners from all over the country and even further afield. It was lovely to meet everyone there in person for the first time after weeks of preparations to ensure everything would run smoothly on the day. However, unfortunately as I am learning, sometimes no matter how much you prepare there are always things out of your control (like a projector that does not want to cooperate and causes a 20 minute delay to the start of the meeting!).
We also hosted a table at the Patient Voice in Cancer Research ‘Dragon’s Den’ event in Galway on the 25th February 2020. This brought the POI management team members and those with a lived experience of cancer together, with the goal of co-designing a patient involvement plan for the POI programme, based on the insights and experiences of those around the table. On the following day, POI partnered with CÚRAM, the SFI Research Centre for Medical Devices at NUI Galway and Galway Film Centre, in this year’s Science on Screen programme, which will commission a short scientific documentary focused on cancer research. Members of the POI management team, together with patient advocates and researchers, shared their insights with an audience of filmmakers, with the focus of the proposed documentary film being on precision oncology. The pitches put forward by filmmakers are currently being shortlisted, and hopefully the successful candidates will be able to shoot the footage in early 2021. That same day, I manned the POI booth at the Irish Association for Cancer Research (IACR) Annual Conference, and I really enjoyed engaging with the researchers, discussing their current work and providing them with information on POI.

Manning the POI booth at the IACR with POI’s COO Jessica Ralston
So, the going was good and then everything changed. On the 12th of March we all headed home and have remained there since. What is the new reality of continuing to set up the programme remotely? To be honest, things have been running surprisingly smoothly thanks to modern technology and the virtual office environment is working well for us. On the operations side, our work is getting done, grant applications have been submitted and our meetings are still going ahead via Zoom in the comfort and safety of our homes.
An insight into our virtual POI management meetings
The clear negatives are that wet lab-based work has ground to a halt and it is unclear when this may fully resume. Recruitment of new hires to several projects has also been delayed due to Covid19 and this could mean that projects might be running behind before they can even start. Significant milestones have been set out for this programme and these delays could of course impact on the timeline for achieving these. The ban on holding events means that we unfortunately cannot plan any networking or team-building activities for newly hired researchers to meet the POI operations team or other researchers involved with the programme. These events are vital for building relationships and creating a sense of community within the programme so we will need to explore other ways of achieving this remotely.
However, on the positive side, in the overall scheme of a five-year project, any delays at the start of the research projects should not cause too many issues and we will still have plenty of time to get the work done down the line.
Now we must adapt to the current situation and continue to plan ahead. However, it is hard to know what future planned events will look like if social-distancing rules must apply. This is especially true for patient and public engagement activities where some attendees may belong to high-risk groups. Perhaps events such as these will need to be put on hold until a COVID-19 vaccine is available. As we can no longer host seminars in person, we have been forced to think creatively and explore innovative ways to engage our audiences remotely. We are currently planning a virtual tour of our industry partner’s labs and facilities, as well as a virtual seminar series. These are things that we probably would have never considered doing if it wasn’t for the new social-distancing restrictions and one clear benefit to working in this way is that the events are accessible to a wider audience as people can join in from home. So, keep an eye out for the invites to these virtual events as they are sure to be excellent.
So luckily, we are able to continue working and making progress in establishing the programme and we are very thankful for this. If we have any personal regrets though it would have to be that we didn’t take out shares in Zoom last year!
Promises of Tissue based imaging in Cancer research and Diagnostics
Transition to the Age of Digital Pathology
When a cancer patient first comes in contact with the clinician, a plethora of tasks sparks in their mind. These include proper diagnosis, selecting the best treatment option, monitoring treatment response, and lastly following up for recurrence of disease. To establish a diagnosis, biopsy tissue materials are often examined under the microscope by a histopathologist. In traditional practice, histopathologists examine glass slides of biopsy tissue under the microscope and characterise specific features relevant to the disease. Over recent years, cancer diagnosis has gone through a significant change due to a substantial improvement in computational power. Radical development of novel high-throughput imaging tools and image analysis algorithms has facilitated the gradual transition of manual histopathological analysis of traditional microscopy to digital pathology. Digital pathology involves digitising histopathological tissue slides into high-resolution images by scanning the slides with whole-slide scanners, and afterwards analysing the digital images with computer-aided image analysis software. After the slides are digitised to computer images, they can be viewed by a pathologist on a computer monitor, where the image can be magnified and navigated spatially in much the same way as standard microscopy. These scanned images represent the accurate depiction of the tissue slide, and in most cases, offer much more ease and flexibility in detecting complex morphological features of diagnostic or prognostic value. Image analysis software is then used to analyse those features of interest, and extract quantitative data from them that are generally much more accurate and reproducible. Figure 1 shows different steps in manual and digital pathology workflow.
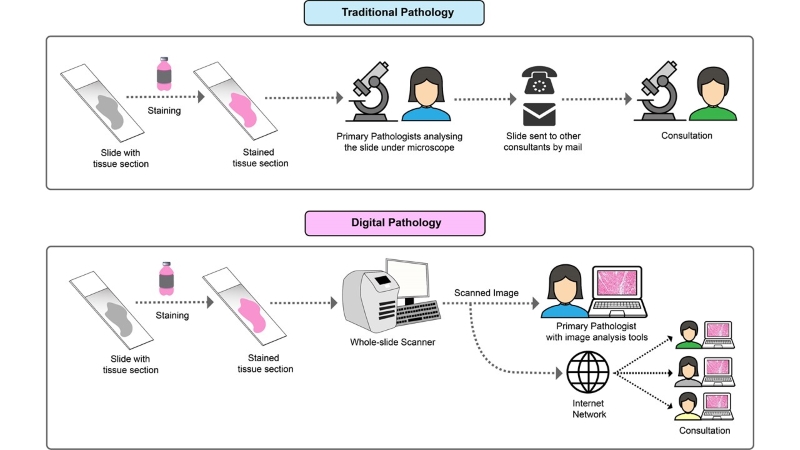
Figure 1: Comparison between the workflow of traditional and digital pathology
For visual assessment of the otherwise transparent tissue samples, the tissue sections go through staining procedures. There are various staining methods used for staining histopathological slides. For example hematoxylin and eosin (H&E) staining, which is used as the routine staining for histological slides, and provides a general overview of tissue morphology. But when pathologists or researchers need to look into more specified features such as presence or absence of specific molecular biomarker (protein/RNA) in cells, other specialised staining techniques like Immunohistochemistry (IHC) and In Situ Hybridization (ISH) are used. IHC uses targeted antibodies to detect a specific protein antigen and ISH uses complementary DNA or RNA strand to localise a particular nucleic acid sequence in the tissue. These antibodies and nucleic acids are normally linked with enzymes or fluorescent dyes, which makes the target visible under the microscope upon binding. Examples of scanned images of some tissue sections with IHC staining can be seen in the figure.
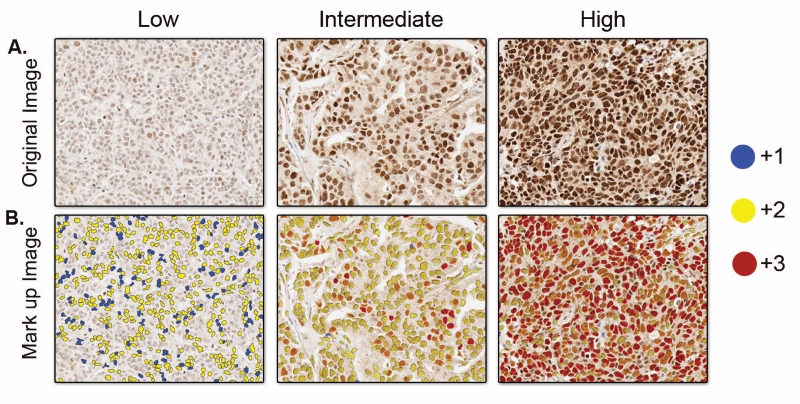
Figure 2: A. Scanned images of IHC staining on different tissue sections showing different levels of expression of a specific biomarker. More intensity of the staining indicates higher levels of the biomarker expression. B. Mark-up image with stained cells detected and annotated by an automated image analysis tool. Different colors of annotation indicates different threshold of staining intensity, blue (+1) indicates weak staining, yellow (+2) indicates moderate staining, and red (+3) indicates strong staining.
Based on these molecules linked with antibodies, there are two different types of IHC techniques: Chromogenic and Fluorescent. Chromogenic IHC depends on the chemical reaction triggered by the linked enzyme molecule and for fluorescent IHC, it’s the linked fluorescent dye. Traditionally, chromogenic IHC detects a single antigen in a slide. This can be a major limitation as cancer is a very complex molecular disease, and to diagnose or predict a proper treatment outcome, multiple molecular events or biomarkers need to be assessed. To solve this issue, Multiplexed Immunofluorescence (mIF) platforms have emerged as a potent tool as they allows simultaneous detection of multiple protein biomarkers on a single tissue section while preserving tumour material. It allows the opportunity to study different components of the tumour microenvironment, providing a better understanding of the cross-talk between the host and the tumour. While not yet used routinely in the clinic, this technique has the potential to dramatically improve diagnosis. Figure 3 graphically represents how multiple targets can be visualised simultaneously in the same tissue with multiplexing.
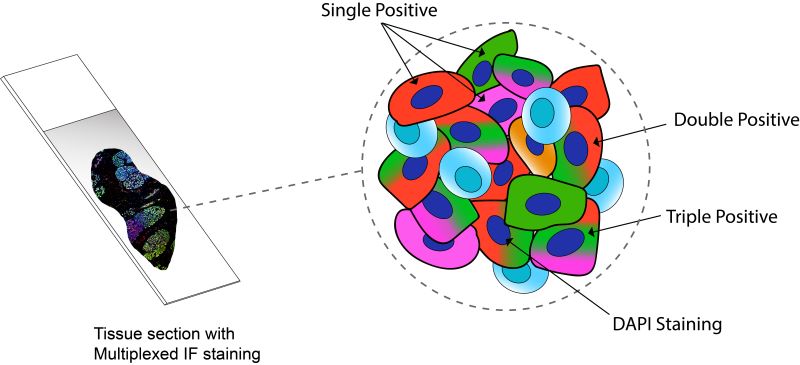
Figure 3: Tissue section with simultaneous staining of four different markers by Multiplexed Immunofluorescence. The schematic is a simplified representation of a magnified portion of the tissue showing cells expressing varied combinations of the three markers and a special fluorescence dye to light up all nuclei in the tissue (DAPI).
Deciphering Tumour Immune Contexture with Multiplex techniques
In recent years, there has been huge interest in the scientific community on mapping the immune architecture of tumours, and tissue-based multiplexed techniques have become one of the most desired tools for executing that. A tumour microenvironment hosts a highly diverse network of immune cell populations which collectively plays a vital role in tumour progression. A lot of different Immune therapies have been developed over the years which attempt to stimulate these immune cells to trigger a desired anti-tumour response, contrary to conventional cancer therapies that target tumour cells directly. Lately a good number of those therapies have also found major clinical success, although in a lot of cases it is still not properly understood why many patients do not respond to these treatments and/or experience immune-related complications. Recent research data suggest that tumour’s response to these immunotherapeutic approaches depends on the presence of specific immune cells in the tumour. These Immune cells surrounding tumour cells commonly referred to as tumour infiltrating lymphocytes (TIL) reflect the host’s immune response against the tumour cells. Numerous research studies have established that the composition of these TILs has significant deterministic value in disease prognosis for various cancer types like Breast cancer, Lung cancer, and colorectal cancer. Along with the presence of different immune cells in the tumour, there are other distinct features of TILs that have potential clinical significance. That is why characterisation or profiling of immune contexture in tumours requires detailed information about the tumour microenvironment, density of different immune cell populations, and their spatial arrangement. Here, spatial arrangement refers to the co-localisation of different immune cells in relation to the tumour cells and among themselves (Figure 4). In a recent study with non–small cell lung cancer, all these spatial features of TILs are found to have predictive value in the likelihood of the recurrence of cancer after chemotherapy. As proper characterisation of TILs demands multiple spatial features to be assessed together, multiplexed staining platforms have emerged as an ideal tool for immune profiling in cancer research. In contrast to other tissue-based imaging tools, multiplexed platforms are the only platforms that allow studying the spatial distribution of multiple immune and non-immune biomarkers and the interactions among them.
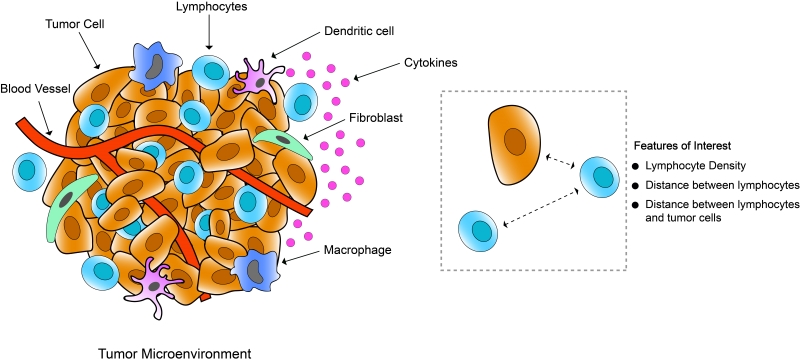
Figure 4: Schematic representation of tumour microenvironment showing tumour infiltrating lymphocytes, epithelial cells, fibroblasts, blood vessels, other immune cells (macrophages, dendritic cells).
Incorporation of Artificial Intelligence in Digital Pathology
Along with the use of high-end imaging tools in clinical and research practice, the need for increasing translational outcome of these tissue-based biomarkers have continued the innovation of new and superior image analysis approaches. Over time in tissue-based biomarker analysis, more and more complex and sub visual features are being explored in clinical practice and academic research. Moreover, the enormous amount of data being generated from these advanced imaging tools has made management of diagnostic workflows increasingly tough. That is why there’s an increasing need to develop tools for pathologists to help them in achieving significant growth in clinical outcome. Artificial Intelligence (AI) comes right into play here. In simple terms, AI is machine-based approaches that emulate human intelligence; its purpose is to try making predictions like an intelligent human does. Integration of AI in image analysis of tissue-based biomarkers offers far more efficient and robust extraction of clinically relevant data from a large patient population and reduces human error to a large extent. It makes a subjective assessment of images quantifiable and extremely reproducible. In recent times, AI approaches for image analysis are shifting towards Deep Learning, which is based on artificial neural networks that imitate the learning process of the human brain. The way deep learning works in pathology is that pathologists feed the network with annotations of a feature in an image (e.g. – blood vessel), and then the neural network learns from those annotations like a human does and later tries to detect those features by itself in the tissue specimen. The more input data it gets, the more precise it gets at identifying the feature of interest and found to match or in many cases perform even better than humans.
There are still quite some challenges to integrating AI in regular clinical diagnostics exclusively. Nevertheless, the potential is boundless. We are in an age where treatment strategies are more and more being tailored to individual patients. Since advanced tissue-based imaging technologies offer ways of acquiring and assessing patient-specific clinical data with far more accuracy and precision than usual, these technologies are now growing immensely in popularity and demand in histology labs, hospitals, universities, and pharmaceutical companies worldwide. Many of them are leaning towards digitising their entire workflow. Over the next ten years, progress in this field will undoubtedly transform the field of oncology and related medical diagnostics. Ultimately, given the phenomenal rise of machine learning and AI, one can only imagine the sensational advancements that will be uncovered in the field of medicine in the next decade.
Author: Chowdhury Arif Jahangir, POI PhD student
 After completing a Master's degree in Molecular Biotechnology under a joint program by Karolinska Insititute and University of Skövde (Sweden), Arif received a research assistantship at Karolinska institute. There Arif worked on investigating the molecular mechanism behind antioxidant induced lung cancer metastasis. After completing the project, Arif joined the Cancer Biology and Therapeutics Lab at UCD as a research assistant and worked under the OPTi-PREDICT project funded by SFI. Arif received his 1st class undergraduate degree (BSc) in Biotechnology from the University of Rajshahi (Bangladesh).
After completing a Master's degree in Molecular Biotechnology under a joint program by Karolinska Insititute and University of Skövde (Sweden), Arif received a research assistantship at Karolinska institute. There Arif worked on investigating the molecular mechanism behind antioxidant induced lung cancer metastasis. After completing the project, Arif joined the Cancer Biology and Therapeutics Lab at UCD as a research assistant and worked under the OPTi-PREDICT project funded by SFI. Arif received his 1st class undergraduate degree (BSc) in Biotechnology from the University of Rajshahi (Bangladesh).
In May 2020, Arif started his Ph.D. with POI, under the supervision of Professor William Gallagher. His project aims to explore the interplay between master transcriptional regulators and immune cells within the tumor microenvironment of breast cancer. His project is co-funded by the Irish Cancer Society.
Doing a PhD during a global pandemic
The first mention of the virus was during lunchtime in the lab on a dull January afternoon. This was followed by a few concerned nods and a light debate before someone mentioned Netflix’s new show and the conversation moved along. Little did we know in a mere few months, a global pandemic would be announced and we would be shutting up shop. At this stage I was only six months into my PhD.
Rumours of lockdown started to circulate on a Wednesday morning amongst the research teams. Later that day, an official email was circulated confirming the closure of the lab until further notice. This was met with a wave of anxiety from my lab group. How would our work get done? What if one of us got seriously sick? A lab meeting was subsequently called and following some encouraging words from our supervisor, we hugged goodbye and parted ways not knowing when we would meet again. As I walked home my mind wandered to our charity partner NBCRI and how vital funds would be raised during this time. I also thought of those with BreastCheck appointments or those receiving treatment and how these people would be accommodated.
My mum, a natural worrier, wanted my sisters and I home once a nationwide lockdown was announced. I am part of a large family of six, the eldest of three sisters, mum, dad and of course our two dogs. It had been a good few years since we had all lived under the same roof so as you can imagine, adjusting to this was a challenge. There were arguments over the internet, noise during video calls and even who ate the last piece of cake put aside for someone else.
While initially I found it hard to be productive during such an uncertain time, developing a routine over the first few weeks helped me adapt to the change in my working day. Our weekly research meeting and journal club recommenced in a new virtual format bringing with it some normality. While I missed the physicality of lab work, this new way of working was a welcomed change. I began to do more in-depth reading around my project and worked on my scientific writing- even beginning a draft of my thesis introduction! I found I began to look forward to our group meetings on a Monday but mostly just to see that everyone was doing okay and to talk to someone other than my family! As the weeks went by and restrictions were slowly lifted, I began to wonder if we might actually get back to Galway. This was met with enthusiasm from our supervisor who mentioned a return to lab plan was in the works. Our research meetings became more intense but exciting as we all prepared plans and conducted order lists in preparation for our return.
On the 29th of June, following countless meetings, induction videos and forms, I finally stepped back into the lab after four months of working from home. The “new normal” took some time to get used to with most of us finding the first few weeks tough. Masks became mandatory making it hot and claustrophobic during work while glasses wearers had an additional challenge to face- fog! The lab days were short (due to rotating shifts) and lonely as we were capped at 25% capacity meaning little to no interaction. I think I found the lack of socialising like grabbing a coffee with a friend to rant or enjoying lunch together the hardest part to get used to.
As the weeks progressed, we were rewarded with longer working hours and increased capacity. This made the days easier, as we were able to meet (Two meters apart of course) and chat with each other. Finally, almost six months to the day since the closure of our lab, we had our first in person, socially distanced research meeting. This in itself brought a real sense of normality to my working day as we shared results and gave feedback to each other.
While there are of course still restrictions in place, I feel hopeful that my lab work will continue to be productive as the months go on. Doing my PhD during a global pandemic has been challenging but has given me a fresh perspective on what is important in life. I am grateful for my fantastic research group and supervisor who have been so helpful and understanding during these strange times. Despite the many arguments, I’ve learnt to appreciate my family more, as well as little things like a walk on the beach and brunch with a friends- something taken away from us all.
It is hard to believe that it has been almost a year since the launch of POI and the beginning of my PhD journey. While a difficult year, I look forward to the work to come, and in particular to being part of a large network of individuals striving to make a difference.
Author: Elan McCarthy, POI PhD student

In October 2019, Elan McCarthy started her PhD with POI, under the supervision of Dr Róisín Dwyer at NUIG. Previous work by this group demonstrated the potential of EVs for treatment of advanced breast cancer. Elan’s project is focused on understanding how these vesicles interact with the immune system, and will be critical to successful translation of this approach to the clinical setting. Her project is co-funded by the National Breast Cancer Research Institute.
Could bacteria function as tissue agnostic cofactors for checkpoint blockade immunotherapy?
Written by: Dr. Sidney Walker, POI Postdoctoral researcher
Immune checkpoint inhibitors (ICI) have provided a disruptive breakthrough treatment strategy for some tumours by reversing the immune privileged status that allows them to survive and grow. Unfortunately, objective response rate is low, not exceeding 30%. This low success rate has prompted the search for biomarkers indicative of an improved outcome. The key biomarker discovered to date is the correlation between high mutational burden, and effectiveness of the ICI therapy. Conversely, antibiotic treatment prior to ICI treatment is known to worsen clinical outcomes in patients receiving this therapy.
The human microbiome is considered a de facto organ with functions in digestion and metabolism, including drug metabolism and immune system regulation among many others. This makes it a logical starting point, especially given the effect of antibiotics.
A potential relationship between the microbiome and ICI treatment outcome was confirmed when it was showed that increased microbial diversity in the gastrointestinal tract corresponded with increased success of immune therapy treatment. These findings have been expanded upon again to show that responders and non-responders to ICI can be stratified based on their gut microbiome, with a panel of bacterial taxa associated with the specific outcome being identified in both groups. It must be said that the underlying mechanism for this remains unclear, and that there is yet to be a consensus on these panels of outcome associated bacteria between studies.
Relationships between bacteria and response to ICI targeting cytotoxic T lymphocyte associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have been identified in multiple tumours to date including:
- Non small cell lung carcinoma
- Melanoma
- Renal Cell Carcinoma
- Urothelial Carcinoma
- Small Cell Carcinoma of the Head and Neck
Faecal Microbiota Transplants (FMT) from human responders into mouse models has been shown to improve response to ICIs, which confirms the key role played by the gut microbiome. As such, progress must be made to identify the underlying mechanisms modulating ICI efficacy before this information can be translated into a therapeutic strategy.
The current cutting-edge research into this potential therapeutic avenue shows that not only FMT (containing billions of diverse bacteria), but also specific bacterial taxa cultured and administered to mice have a significant impact on their response rate. The prime example is B. pseudolongum, a key metabolite of which is the nucleoside inosine, found to enhance the effect of anti-PD1 treatment through enhanced activation of anti tumour T-cells. This process is accelerated by the degradation of gut barrier function which is a side effect of immunotherapy. This was observed across a spectrum of tumour types such as colorectal cancer, bladder cancer and melanoma.
It is premature to state whether such effects could be repeated in humans, and considerable research is still required, but results in preclinical models raise the prospect for the following precision medicine treatment strategies:
- At a minimum, point of care sequencing using rapid turnaround tabletop sequencing technology to rapidly stratify patients into appropriate treatment group prior to therapeutic intervention.
- The ideal endpoint would be personalized treatment based on the results of (i) where targeted antibiotics are used to remove antagonists of ICI function, and create a niche if necessary, to be colonized by known agonists of ICI function.
About the Author: Sidney completed a PhD in Bioinformatics investigating the tumour microbiome in 2019, and now works as a post-doctoral researcher in the Dr Mark Tangney's lab in UCC. His project in POI is co-funded by Breakthrough Cancer Research.

Barrett's Biobanking and registry
Written by: Rebecca Anderson
Barrett’s oesophagus is a pre-cancerous condition which involves the development of abnormal cells in the lower oesophagus. This is understood to be caused by persistent acid reflux over a long period of time. The main symptoms of Barrett’s oesophagus are acid reflux and indigestion, and the condition is diagnosed by an endoscopy (a camera test) and histological examination of small biopsies (cell samples) taken during this test by a scientist in the laboratory. Thankfully, less than 1% of people who have Barrett’s oesophagus go on to develop cancer, but it remains important that we monitor patients with Barrett’s for progression of their symptoms, and work to understand better the connection between Barrett’s oesophagus and oesophageal cancer development.
A biobank is a large collection of biological samples and their accompanying medical information which can be used by scientists to do research. The Barrett’s biobank is a collection of tissue samples, blood samples and gastric juice samples from patients with Barrett’s oesophagus who have agreed to be involved in research into the condition. These samples are taken during the patient’s routine surveillance camera tests and are stored in the trinity translational medicine institute in St James’s hospital Dublin until they are needed for research. The Barrett’s biobank is an incredibly important resource for scientists interested in the condition, and allows them to ask questions about how Barrett’s oesophagus develops, and why some patients progress to cancer while others don’t. The power of a biobank is in its size. The more samples involved in asking a research question the more robust the results of experiments are, and we are very grateful to the patients who allow us use their samples.
As well as the Barrett’s Biobank, which allows scientists do laboratory research on the condition, we also maintain the Barrett’s registry, which contains the pertinent medical information of patients diagnosed with Barrett’s oesophagus. The registry is a national effort managed from St James’s hospital in Dublin, and involves hospitals from all over the country. We can use the registry to run audits, which allow us to ensure patients with diagnosed Barrett’s oesophagus are being monitored, and are having regular surveillance endoscopies. We can also use audits to track trends in the diagnosis of Barrett’s oesophagus in Ireland as well as in progression of the condition more generally.
The biobank and registry are powerful tools which allow us to better understand the condition of Barrett’s oesophagus and our patients. The overarching aim of the Barrett’s oesophagus biobank and registry is to improve patient care, whether through laboratory research using biobanked samples or careful monitoring of the condition through registry audit.

About the Author: Rebecca Anderson is the Barrett's Oesophagus Data and Biobank Manager in Beaumont Hospital. Her project in POI is co-funded by the Oesophageal Cancer Fund.
Making public and patient involvement in research a reality in Precision Oncology Ireland
Written by: Dr. Fiona Lanigan
Since the launch of Precision Oncology Ireland (POI) in November 2019, we have always strived to involve cancer patients and their families in our research as much as possible. During the patient forum held at the launch event, participants spoke of how they felt that being involved with cancer research gave them hope, a feeling of some power over their condition, and also the opportunity to give back to research, which in some cases had been instrumental in their treatment and recovery.
However, implementing public and patient involvement (PPI) in research at a practical level is not without its challenges. Depending on the research project, it can sometimes be difficult to understand how best to involve patients in the research in a meaningful and non-tokenistic way. This is particularly true for research projects at an early stage of their development, where the research may be focused on understanding how a particular process drives a tumour cell to grow, rather than developing a specific treatment for it, which would come at a later stage.
Another challenge we came across early on when developing plans for PPI was the fact that the research projects within POI had been defined when the funding proposal was submitted. Obtaining research funding can be a long drawn-out process, and the foundation of the POI research projects had been set out several years previously following extended discussions between academic researchers and partner organisations. Each of the research projects within POI, which are comprised of a mix of early-stage and later-stage research, are co-funded by Science Foundation Ireland plus an industry or charity partner. If the direction of this research was already defined, would it be fair for us to invite patients to be involved at this point, and give of their time, unless there was scope for us to implement the changes they might suggest?
It was with these questions, and more, in mind that we first heard about an initiative led by the Patient Voice in Cancer Research (PVCR). The PVCR was established in UCD in 2016 and aims to facilitate dialogue between patients and researchers so that the lived experience of cancer patients can enrich, inform and shape the research process.
They had planned an event in association with the UK-based National Cancer Research Institute, to be held in Galway in February 2020, called the ‘Dragon’s Den’. The idea behind this format was that researchers would apply to the PVCR to have their proposal or idea heard by a team of ‘Dragons’ – those with a lived experience of cancer who had applied to participate in the initiative.
Who better to advise us on how to involve patients in our research but the patients themselves? We drafted a PPI strategy as a starting point for our discussions, and set off for Galway to present it to the Dragons, hoping that they wouldn’t be too fearsome!
In those golden days pre-COVID, the PVCR events were a hugely social occasion, and we enjoyed the informal interaction with attendees and the buzz of the event, before sitting down with our team of eight Dragons.
We started our pitch by explaining why we as a research programme wanted to involve patients in our research: to improve the relevance of our research to patients, to define areas of unmet need for future research projects, and most of all, to make patients, who are ‘experts by experience’, our partners in research. We discussed the focus and scope of our research projects, and the spectrum of activities we hoped to participate in, from patient engagement (communicating to patients or imparting information) to patient involvement (actively partnering with patients in developing research). We outlined the questions and challenges that we had been grappling with in developing our PPI strategy, most of all the fact that we wanted our PPI activities to be achievable, worthwhile, and mutually beneficial. We then handed the floor to our 8 Dragons for their feedback.
The first key point made was that PPI is a two-way street. We as researchers may often feel that we are asking too much of patients, but they can say no if they do not want to be involved - we should not be afraid to ask. That said, our Dragons wanted this involvement to add value to the research process and not be tokenistic. One suggestion to ensure contributions were meaningful was to include patient representative(s) on the Governance Board, where they could bring not only their lived experience as a cancer patient to the table, but also their life skills and career experience. The difficulties in incorporating PPI into pre-existing projects was acknowledged, but this process is about developing partnerships in the longer term, so that future research programmes in the pipeline take patient input into account from the start. A suggested starting point was to hold a ‘POI research showcase’ where POI research projects were presented to a public and patient audience in an accessible way, allowing them to gain insight into the ongoing work and provide input from their perspective where possible. This may then lead to more in-depth partnerships over time.
The group also suggested practical ways that patients could be involved in promoting the importance of research to the wider public, such as partnering on community engagement events, and participating in the dissemination of research outputs. On a practical level, the importance of adequate compensation to ensure patients are not out of pocket due to participation in PPI activities, and acknowledgement of contributions in grant proposals, research publications or presentations, where appropriate, were highlighted. The point was also made that PPI participants were often a highly pro-active and self-selecting group, and more effort should be made to engage with seldom-heard groups and minority communities by attending or organising smaller local events targeted at these specific groups, or facilitating online events for those unable to travel.
We could not have foreseen that this would be the last time for a long while that we would meet other people to physically sit around a table, and we enjoyed the experience enormously, as well as gaining some insights into how best to map out our PPI strategy. Feedback from the Dragons to the organisers was also very positive, and in general this format of event has a lot to offer in terms of bringing patients and researchers together to facilitate PPI.
Based on the advice received, we have implemented some changes to our programme, outlined below. On top of this are the enforced changes to all our lives following the spread of COVID worldwide, which has brought some surprising benefits in terms of accessibility, as well as the obvious downsides.
- We are planning to hold a virtual ‘POI Showcase’ in 2021, which will be co-developed with patients. This aims to bring POI researchers and patients together to discuss and provide input on aspects of POI research, or proposed projects for the future. We hope that similar events in the future can be held in person!
- We have included patient representation on the POI Governance Board with the appointment of Jan Rynne, a hugely experienced patient advocate who will bring her unique insight to the Board.
- The Dragons suggested that POI should engage with the public by organising lay talks relating to POI research. Since February 2020, we have held 4 virtual public research talks in collaboration with various charity partners and patient advocacy groups.
- Based on the suggestion that more efforts be made to engage with under-represented groups, POI have worked together with the leaders of selected minority community groups to co-create a programme of engagement activities for Science Week 2020, funded by Science Foundation Ireland (For more info, see http://www.precisiononcology.ie/engagement/invisiblespectrum/) . We hope that this will be a starting point for further activities aimed at engaging minority groups in our research.
- We have also included in our publication policy that the contribution of PPI participants to research projects should be acknowledged in all research outputs going forward.
We would like to thank all of the PVCR Dragon's who contributed their insight and patient experience to help develop our PPI plan. If you are a patient or interested stakeholder (patient advocate, family member, carer, health care professional, policy maker) interested in sharing your experience and expertise and becoming involved in PPI in Precision Oncology Ireland, contact poiadmin@ucd.ie, or contact patientvoicecancer@ucd.ie to find out more about the Patient Voice in Cancer Research.
About the Author: Dr. Fiona Lanigan is the Communications, Dissemination and Public Engagement Manager with Precision Oncology Ireland

The importance of nutrition in cancer care
Precision nutrition aims to tailor dietary advice to fit the specific needs of an individual1. This is particularly important for patients undergoing cancer treatment, in order to minimise weight loss and malnutrition. Malnutrition and weight loss, affects up to 56%2 and 70%3 of cancer patients respectively. Both factors actually lower cancer treatment tolerance, leading to reduced chemotherapeutic dose3. High-protein, high-energy diets are often required to circumvent this weight loss. This may seem at odds with current healthy eating guidelines, however, cancer is a unique scenario, wherein energy and nutrient requirements can be quite high, as cancer really affects metabolism, and appetite is often poor4.
Despite increasing awareness that specialist dietitians should be included as core members for many cancer types, inadequate resourcing means that most tumour MDTs have limited or ad-hoc dietetic service provision at best. It is estimated that between 1/3 to 2/3 of malnourished cancer patients receive no nutritional advice5. A study conducted in Irelands 9 specialist oncology centres by Lorton et al., which included 200 patients, the majority of which had gastric or hepatobiliary cancer, found that referrals to a dietician in both inpatient and outpatients were “reactive rather than proactive”, in response to weight loss. 45% of dietician referrals should have been made sooner according to the oncology dietitian while only 1/3 of patients received nutritional screening2. A survey of malnutrition in Ireland conducted by Sullivan E.S et al., in 1073 cancer survivors shows similar results with only 39% of those surveyed having been assessed by a dietician. This is despite 45% of this group having diet related problems and 89% of responders saying diet was ‘very/extremely’ important in cancer care, highlighting just how many of these patients need and want dietary guidance6 .
However, lack of referral is not the only hurdle cancer patients must overcome to receive sound nutritional advice. Currently, there is only 1 dietician per 4500 cancer patients in Ireland making access limited6. According to Professor John Reynolds of St James’ Hospital and Trinity College Dublin, a leading expert in Gastric Cancer, it would cost €1.6 million to hire the necessary oncology dieticians and provide services for the 10,000 cancer patients in need of nutritional screening each year. This cost, however, could be off-set by savings made in the cost of treatment, as malnourished cancer patients have longer hospital stays, suffer increased chemotherapy toxicity and dose reduction, frequent hospital readmission and reduced quality of life (QOL)7,6. Indeed, a landmark study in Australia determined that the cost benefit of screening for malnourished patients outweighed the cost of putting these screening systems in place8.
The main goal of cancer nutrition is improvement or indeed maintenance of nutritional status, with a particular focus on maintaining lean mass9. Lean mass refers to body weight excluding fat mass (concentrated in adipose tissue), wherein skeletal muscle mass is a large component. This will reduce treatment associated side effects, aid post-surgery recovery and improve patient’s QOL. Cancer patients are one of the most malnourished group in the hospital setting8. Many patients have nutrition ‘barriers’ that prevent them from eating normally such as anorexia, nausea, vomiting and difficulties in swallowing2. Cancers of the digestive tract cause the most severe weight loss, while some like breast cancer can cause weight gain or no changes in weight at all2. Work conducted by Dr Oonagh Griffin in St Vincent’s University Hospital Dublin demonstrated that unintentional weight loss and resultant malnutrition can be easily missed in patients who were obese prior to diagnosis10. When creating a nutrition plan for these patients, it is important to take their individual symptoms, cancer type and personal tastes into account. For those with difficulty swallowing, softer foods and things like soup which are easy to swallow are key for getting in calories and nutrients. Resources recommend eating smaller more frequent meals rather than 3 large meals which can lead to people feeling full quickly. A patient centred cookbook created by Dr Aoife Ryan and Eadaoin Ni Bhuachalla called ‘Good nutrition for cancer recovery’ is an amazing resource full of high protein, calorie rich recipe ideas (Figure 1). It also includes advice for patients at the beginning of the cookbook for each barrier they may face, for example ‘Avoid greasy, spicy and sugary foods with strong odours’ for those with nausea and vomiting11.

Figure 1: Good nutrition for cancer recovery
When your appetite is poor, achieving adequate energy and protein intake is challenging. Protein is essential for maintaining lean mass, tissue repair and also helps patients withstand the effects of chemotherapy and prevent further weight loss. Protein requirements for healthy individuals is 0.8g/Kg each day, this increases to 1.2-1.5g/Kg per day for cancer patients12. Therefore, a woman weighing 60kg would need around 90g of protein a day. Or a 90kg man would require 135g per day. To put that in perspective, Figure 2 presents food portions that deliver 30-40g of protein. A medium sized chicken breast (100 grams), for example, provides 31 grams of protein. However, many patients do not meet the protein requirements for healthy individuals, not to mind cancer recommendations, due to treatment and/or symptom related side effects. Protein is particularly important for those with cancer cachexia. Cancer cachexia refers to the rapid unintentional weight loss, particularly associated with significant loss of lean body mass, seen in many cancer patients. Patients with cachexia have an altered metabolism which shifts away from muscle building towards the breakdown of muscle13. A study conducted in UCC in foregut cancer patients found the prevalence of cachexia to be as high as 62%14. It is a complex response to malignancy and/or cancer treatments of which there are few treatment options. However, maximising protein and energy intake is paramount for these patients to minimise its affects.

Figure 2: What 30-40g of protein looks like in food. It is equivalent to ~ ¾ L skimmed milk, 5 boiled eggs, ¾ chicken breast or ~ 3-4 oz of cooked mince.
As well as ensuring adequate levels of fat and protein, vitamins and minerals are an important part of any diet but especially cancer patients. As cancer can adversely affect appetite and eating habits, many cancer patients may not get their recommended amount of essential vitamins and minerals15. It is estimated that 72% of cancer patients are vitamin D deficient16, compared to 20% of the general population17. Vitamin D is obtained through vegetables (vitamin D2), oily fish, fortified foods and the exposure of skin to sunlight (Vitamin D3). It is responsible for a healthy skeletal system and helps regulate calcium in the body15. The VITamin D and OmegA-3 TriaL (VITAL) was a large randomized clinical trial in 25,871 men and women that investigated whether dietary vitamin D3 or fish oil supplementation reduced the risk of developing cancer. While vitamin D supplementation did not significantly reduce cancer incidence, post-hoc analysis of the 5-year primary prevention study demonstrated a possible reduction in fatal cancer with vitamin D supplementation, depending on subject’s body weight18. Advanced cancers (metastatic or fatal) were significantly reduced following vitamin D supplementation if the subjects were not overweight or obese. However, it is important to note that evaluating Vitamin D status in the acute phase of inflammation or cancer is a challenge. C-reactive protein, a common marker of inflammation, is associated with lower serum 25(OH)D (vitamin D) levels and therefore may not be an accurate reflection of vitamin D status. In addition, 90% of vitamin D is bound to vitamin D binding proteins so measuring free serum levels may not give the most accurate result19,20. More work is needed in this area to improve our understanding of micronutrient status, including sensitive methods of measuring Vitamin D, in the presence of cancer.
Despite the importance of adequate vitamin and mineral intake, specialists do not advise exceeding the recommended daily dose. If a patient is struggling with food intake a multi-vitamin can be taken but it is imperative to consult with the oncologist before taking any new medication. As vitamins and other herbal supplements are not well regulated, it can be difficult to know what you are truly getting. Herbal remedies might seem harmless but some such as St John’s wort has been shown to decrease the efficacy of certain medications including chemotherapy21. As dietary advice for patients can be lacking, it is natural for those with cancer to seek information online. While there can be many great resources for patients, such as the cookbook I mentioned above as well as the Memorial Sloan Kettering integrative cancer centre, there is also a lot of false information out there. Extreme diets that cut out entire food groups and claim to cure cancer can do more harm than good. It is best to consult your doctor before making any drastic changes to your diet.
Receiving a cancer diagnosis can be a devastating time in a person’s life. Patients are often given lots of information at once and it can be difficult to take it all in. Diet may not even enter a patient’s mind until they start to experience symptoms that affect their eating and by then it may have already affected their weight. Conversely, adopting dietary changes may be at the forefront of some people’s minds as it may be the only component of treatment they feel can choose or control. Dietician referral should be integrated into first line cancer care, not just after surgery or when major weight loss has occurred. This could help save the lives of many patients, giving them the accurate information they need to make well informed decisions about their health. To make this possible, nutritional screening, well defined referral criteria and education and resourcing of relevant dietetic staff is necessary to bring about the necessary changes and improve the lives of cancer patients.
About the Author

Rianna started her PhD in January 2020 with Professor Helen Roche in UCD. Her project is focused on finding a biomarker for individuals with cancer cachexia and sarcopenia in the hopes of determining who will benefit from the drug Anamorelin by Helsinn.
Authors: Rianna McElroy (1), Oonagh Griffin(1)(2) & Helen M Roche (1)(3).
Affiliations:
1. School of Public Health, Physiotherapy and Sports science. UCD Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, 4 Dublin, Ireland.
2. National surgical centre for pancreatic Cancer, St Vincent’s University Hospital, Dublin 24, Ireland.
3. Nutrigenomics Research Group, UCD Institute of Food and Health, UCD Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, 4 Dublin, Ireland.
References:
- Juan de Toro-Martín BJA, Jean-Pierre Després,Marie-Claude Vohl. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic syndrome. Nutrients; 2017. p. 913.
- Cliona LM, O G, K H, F R, G S, N G, et al. Late referral of cancer patients with malnutrition to dietitians: a prospective study of clinical practice. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2020;28(5).
- Louise Daly, Ross Dolan, Derek Power, Éadaoin Ní Bhuachalla, Wei Sim, Marie Fallon, Samantha Cushen, Claribel Simmons, Donald C. McMillan, Barry J. Laird, Aoife Ryan. The relationship between the BMI‐adjusted weight loss grading system and quality of life in patients with incurable cancer. 2020;11(1):160-8.
- Maria Rohm AZ, Juliano Machado,Stephan Herzig. Energy metabolism in cachexia. EMBO reports: EMBO reports; 2019.
- Xavier Hébuterne EL, Mauricette Michallet,Claude Beauvillain de Montreuil,Stéphane Michel Schneider,François Goldwasser. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN Journal of parenteral and enteral nutrition. 2014;38(2).
- ES S, N R, E K, A K, JV R, J F, et al. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clinical nutrition ESPEN. 2021;41.
- Kim DH. Nutritional issues in patients with cancer. Intestinal research2019. p. 455-62.
- AG B, JM L, BL S. Using a public hospital funding model to strengthen a case for improved nutritional care in a cancer setting. Australian health review : a publication of the Australian Hospital Association. 2013;37(3).
- Norleena P. Gullett VM, Gautam Hebbar, Thomas R. Zieglerc. Nutritional Interventions for Cancer-induced Cachexia. Curr Probl Cancer.2012. p. 58-90.
- Neil B, Oonagh G. Nutritional considerations for the management of the older person with hepato-pancreatico-biliary malignancy. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2021;47(3 Pt A).
- Good Nutrition for Cancer Recovery – a nutritional resource for the treatment of cancer‐induced weight loss - Ní Bhuachalla - 2016 - Nutrition Bulletin - Wiley Online Library. Nutrition bulletin. 2021;41(2):151-4.
- P R. Nutrition in Cancer Patients. Journal of clinical medicine. 2019;8(8).
- T A, KP T, A R, H M, K T. Cancer cachexia, mechanism and treatment. World journal of gastrointestinal oncology. 2015;7(4).
- LE D, ÉB NB, DG P, SJ C, K J, AM R. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. Journal of cachexia, sarcopenia and muscle. 2018;9(2).
- Kurt A. Kennel MTD. Vitamin D in the cancer patient. Curr Opin Support Palliat Care.2013. p. 272-7.
- Ali A, Berna KE. Vitamin D deficiency in cancer patients and predictors for screening (D-ONC study). Current problems in cancer. 2019;43(5).
- Abigail Cowan RPMaAM. Treatment of Vitamin D Deficiency in Adults. Wirral University Texas; 2020.
- PD C, Division of Preventive Medicine BaWsH, Harvard Medical School, Boston, Massachusetts., WY C, Department of Medical Oncology DFCI, Harvard Medical School, Boston, Massachusetts., ON A, A H, et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Network Open. 2020;3(11).
- MC S, TW F. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutrition research (New York, NY). 2015;35(2).
- DANIEL D. BIKLE EG, BERNARD HALLORAN, MARY ANN KOWALSKI, ELIZABETH RYZEN, JOHN G. HADDAD. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. The Journal of Clinical Endocrinology & Metabolism. 2021;63(4):954-9.
- Josefson D. St John's wort interferes with chemotherapy, study shows. BMJ. 2002;325:460.
Cancer and Stress: Targeting stress responses in cancer cells
We think of cancer cells as fast-growing, highly adaptable cells that can withstand and sometimes even thrive in extreme conditions. When tumours grow fast, the blood vessels that supply oxygen and nutrients to the tumour can’t keep up.
This can be a stressful scenario for a cell. Despite these stress-inducing scenarios, tumour cells continue to grow. Similarly, when tumour cells are treated with traditional therapies, like chemotherapies, these drugs should kill cancer cells but many cancer cell types display excellent abilities to avoid drug-induced cell death. One may even say cancer cells have developed a tremendous ability to cope with stress. So how do they do it?
Since joining the Precision Oncology Ireland team, my research has focused on one of these potential pro-survival mechanisms. We study a protein called Inositol-requiring enzyme 1 (IRE1) and we are particularly interested in how this protein functions to promote cancer cell survival, phenotype, and oncogenic behaviour. IRE1 is located in the endoplasmic reticulum (ER), a vast cellular organelle that assists in the processing of newly made proteins.
The proteins in a cell are made from messenger RNA (mRNA). The ER ensures that cellular proteins are made properly and have the correct structure necessary for them to perform their designated function/s. If a cell becomes stressed, e.g., if nutrients or oxygen supplies are low, newly synthesised proteins can often be made incorrectly. IRE1 senses these badly made proteins as they accumulate in the ER and it initiates a signaling pathway that acts to restore protein balance.
In its simplest terms, I like to think of IRE1 as behaving like a Pacman when it’s active. Active IRE1 acts to degrade mRNAs by breaking them up, much like a Pacman would if it continued to open and close its mouth and mRNAs were its dinner. IRE1 is a bit more specific about what it eats for dinner, but hopefully, you get the general idea! By degrading specific mRNAs, IRE1 acts to decrease protein burden in a cell. Additionally, one of the best-described functions of IRE1 is its cleaving of XBP1 mRNA. When 26bp of XBP1 mRNA is cut out, the two pieces of RNA that are left behind are re-ligated and this mRNA is translated into a highly active transcription factor called XBP1s. XBP1s acts to switch on genes that help to restore protein balance.
In healthy cells, the IRE1 signalling pathway is necessary for the cell to adapt to, or overcome, a stressful scenario. However, we think that cancer cells often utilise this same pathway to ensure that they can survive any stress they may encounter. Our group has previously shown that in multiple cancers IRE1 and its downstream signalling pathway is active and when some of these cancer cells are treated with certain chemotherapies, subpopulations of cells may capitalise on the IRE1 pathway to evade drug-induced cell death.
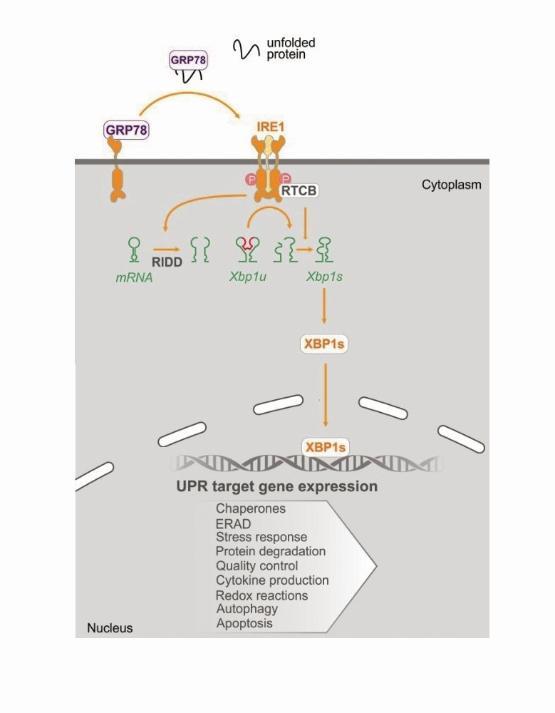
These observations have encouraged us to investigate the benefits of targeting IRE1 in cancer, by inhibiting its ability to degrade mRNAs (effectively shutting down its Pacman-like behaviour). Our research currently investigates in more detail the roles of active IRE1 in cancer cells as well as the therapeutic benefits of targeting IRE1 in cellular models of triple-negative breast cancer and pancreatic cancer. We believe that modulating IRE1 activity may be advantageous in the successful treatment of these cancers.
Author: Dr. Claire Robinson

Dr. Claire Robinson received a B.Sc and Ph.D. from University College Dublin, Ireland. She completed a Post Doctoral Fellowship at the University of Toronto, Canada. Claire currently works at the Apoptosis Research Centre in NUI Galway.
Precision Medicine: The key to improved cancer outcomes in poor-prognosis cancers?
Precision, or personalised medicine in oncology refers to treating a patient’s cancer based on the specific makeup of their cancer and not by applying a one-size-fits-all approach. This often involves using newer ‘targeted’ treatments and foregoing traditional chemotherapy approaches. As an oncologist, I have seen firsthand the difference this has made to select patients, not just with regards to their better outcomes from treatment, but also due to the improved side effects from these treatments.
Our project is focused on patients with advanced gastrointestinal cancers (including liver, stomach, oesophagus, and pancreas). Unfortunately, a large majority of the patients that present to our clinic do so at an incurable stage, and outcomes remain poor with traditional treatment pathways. We seek to examine how applying a precision medicine approach to patients with advanced gastrointestinal cancers changes the treatment plan and overall outcome. We are also particularly interested in recognising any common changes in the makeup of patients' cancers that might lead us to new potential targets for treatment.
So how do we do this? Genomic sequencing allows us to examine the makeup of the cell by ‘reading’ the DNA, which makes up our genes. Genes are essentially instructions for our bodies. By doing this we can identify if there have been any abnormal changes in the genes, also called mutations. Mutations may be responsible for the development of cancer or can drive the behaviour of cancer. If we can identify what is driving the growth of cancer, we may be able to use an existing treatment that can act on that exact change and stop the growth, or potentially develop a new treatment based on that change.
There is one problem with this approach, however. Genomic sequencing produces a lot of data. Also, not all mutations that we identify are responsible for causing cancer. What we want to do is to try and pick out patterns common to cancers, indicating this might be driving the growth of that cancer. One way of overcoming this is to examine people who have an unusual presentation of their cancer, for example at a younger age than expected or had an unexpected response to cancer treatment, either good or bad. We call these ‘extreme phenotypes’. By studying extreme phenotypes we improve the likelihood of discovering a meaningful, cancer-driving mutation that might prove useful as a target for treatment to a larger group of patients
Preliminary work on our project commenced before the Covid- 19 pandemic and it would be remiss to talk about our research study without mentioning how the pandemic has affected it. Before we can recruit our first patient, there is a necessary but lengthy process to ensure the study meets the expected ethical, data protection, and clinical standards. As the medical and indeed wider community were grappling with the frightening new reality of SARS-CoV2, time and resources were appropriately focused on clinical care.
Processes for approval in the relevant committees were lengthier (many of these committees are made up of healthcare professionals and others who are volunteering their time to participate) and as oncologists, we were focused on implementing new patterns of care and familiarising ourselves with how to treat Covid-19. Following the initial stages of the pandemic, however, the oncology community has advocated for continuing research studies and clinical trials - these are essential for providing access to new treatments to our patients and in driving forward our progress in the treatment of cancer.
There are some advantages to precision medicine during a pandemic. Many of these targeted drugs have a different way of working than traditional chemotherapies and as such have a different set of side effects. One thing we worry about with traditional chemotherapies is their ability to affect the body’s immune system and hamper its ability to fight off infection. Naturally, this is a serious concern when in the midst of a pandemic, as it may put patients at increased risk of a severe disease course from Covid-19. In many cases, these targeted treatments are not as immunosuppressive as chemotherapies.
Many, but not all, of the targeted treatments, are taken orally. This can reduce the frequency our patients attend the hospital in person, reducing the risk of contracting the virus. The other hope with a precision medicine approach, although not guaranteed, unfortunately, is that if we can offer targeted treatment, this will be more effective at treating an individual’s cancer and in turn lead to healthier patients who are more able to withstand the effects of Covid-19.
Our project run by Prof Maeve Lowery, Professor of Translational Cancer Medicine in Trinity College Dublin, will enable upper gastrointestinal cancer patients in Ireland with extreme phenotypes to have genomic sequencing of their cancer performed. We hope to tailor treatment plans for those patients based on their cancer genetic makeup and also to examine patterns within all of our patients to identify new targets for treatments. This is a pilot project, to see how feasible the approach is in a small number of patients, but we hope if successful to expand this approach to more patients across Ireland.
Author: Lynda Corrigan

Author's Note: I am a Medical Oncologist currently pursuing an MD, Doctor of Medicine, a degree in the field of oncogenomics under the auspices of Prof. Maeve Lowery. I have a keen interest in the area of oncogenomics and upper gastrointestinal cancers. My training has provided me with extensive experience in clinical research which has given me a robust understanding of the practical implications of precision medicine for oncology patients. Precision Oncology Ireland provides a unique opportunity to build partnerships, share expertise and enhance output within this exciting field, with the ultimate goal of advancing oncology diagnostics and treatments for cancer patients.
Blood Cancer Biobank Ireland - supporting translational and clinical research
Written by Tatiana Cichocka
Blood cancers such as acute myeloid leukaemia (AML), multiple myeloma (MM), and chronic lymphoid leukaemia (CLL) are very rare and complex diseases. They are very variable, or heterogenous, at the genetic, cellular, and microenvironment levels, making both the treatment development and decisions on treatment very difficult. Although some advances have been made over the last decades in treatment for some of these cancers, many still remain difficult to treat.
Biobanks are a crucial part of research. This article sums up the role of biobanks in cancer research in Ireland.
In order to come up with better treatment for blood cancers, a lot of translational and clinical research is required. This involves screening the proposed treatment on a large set of patient samples to find out whether it is widely effective. Typically, the number of patient samples one can obtain is the bottleneck to these studies, as blood cancers are rare.
Blood Cancer Network Ireland (BCNI) alleviated that problem for haematology researchers in Ireland by setting up a national blood cancer biobank. A biobank is a collection of biospecimens, such as biopsies from tissues. In the case of the blood cancer biobank, it is a collection of bone marrow and blood samples from blood cancer patients. For storage, the samples need to be processed, which has to be done by highly trained staff using standard operating procedures (SOPs). Moreover, throughout the sample processing, many quality control steps are taken to ensure the highest sample quality. All procedures are well documented and fully GDPR compliant.
I am the manager of the Blood Cancer Biobank and my job is to manage (and partially carry out) the process starting with the collection of the samples at the hospitals to its storage and its final usage in research projects. My work starts when I get a call from the hospital that a patient suspected of having blood cancer has offered to donate a sample to the biobank. If the patient consents, a sample of bone marrow and/or peripheral blood is taken for the biobank alongside their clinical diagnostic sample. Samples can also be collected after the completion of treatment and upon relapse from the same patient. I either pick up the sample myself, if it's from our local hospital, or if it was collected in a hospital in another city, the hospital staff couriers the sample to the biobank lab. I will then process the arrived samples in the biobank laboratory, by separating the samples into a range of fractions. These include the blood plasma, mononuclear cells (most white blood cells that include the cancerous cells), and bone marrow stromal cells. These are then stored in ultra-low temperatures until needed by researchers.
The stored samples are highly annotated on the biobank’s database. The annotation includes the patient’s clinical data (precise diagnosis, treatment details, and the response to the treatment) and biospecimen data (cell concentration, number of tubes frozen down, and the precise location of the sample). It is also my job to be sure that all sample tubes (which are labeled with individual QR codes) have their storage location recorded in the biobank’s data management system, and this is annotated with the patient’s clinical information.

The samples we store can support both translational and clinical research either through prospective sample collection or through the use of already collected samples. Any researcher with an ethically approved study can apply for the use of biobank samples. When studies get approved for use of biobanked samples, it is my job to retrieve the samples from storage and provide them to the research team.
Currently over 500 patient samples are stored within the Blood Cancer Biobank, equalling about 15,000 tubes. This was collected by 7 different hospitals across Ireland, which are part of BCNI. As a result, even if a researcher is looking for a specific subtype of blood cancer or patients that received a certain kind of treatment, there should be a choice of samples for that study.

One study that biobanked samples were used for was to determine if an in-vitro assay could be set up to determine a patient’s response to the standard-of-care chemotherapy used to treat AML. 36 AML patient samples were tested (which were previously biobanked), and the clinical response of the patient was obtained from the treating hospitals. It was found that by keeping the AML cells together with the stromal cells of the bone marrow, it was possible to predict the patient’s response to the drug treatment within days as opposed to weeks in the clinic. This could be clinically relevant, as the results may allow clinicians to change the treatment approach if they know that the patient will not respond to the treatment.
Another study the biobank is currently involved in looks at the response of chronic lymphocytic leukaemia (CLL) patients to COVID vaccines. CLL patients, due to impaired B cell production, are immunocompromised and they are known to have an incomplete response to immunization. The study aims to understand better the actual efficacy of COVID vaccination in these patients, and whether booster vaccines can improve it.
A blood cancer biobank is an invaluable tool for the haematology community in Ireland. It can provide a solution to conducting more thorough studies in a shorter timeframe than otherwise would be possible, leading to improved treatment options for future blood cancer patients.
Author: TATIANA CICHOCKA

Tatiana is the manager of the Blood Cancer Biobank Ireland, a national effort focused on coordinating and standardising blood cancer biobanking activities at sites in Galway, Dublin, Cork, Limerick and Waterford. The biobank is co-ordinated by the Blood Cancer Network Ireland, led by POI Investigator Dr. Eva Szegezdi, and based in the National University of Ireland, Galway.
Gene therapy in cancer treatment: how nanomaterials solve delivery issues
Written by Dr. Anna Bogdanska
According to the World Health Organisation (WHO), cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020. In Ireland, 1 in 4 deaths is caused by cancer. Cancer occurs when there is a disruption to the cell growth and function caused by damage (mutation) to genes within those cells. Gene therapy is an emerging form of treatment where genetic material is delivered to defective cells to either insert a missing gene, correct, or delete a mutated gene or to deliver a therapeutic gene. Despite the immense potential of this type of therapy, there are many obstacles preventing novel gene therapy formulations from advancing into clinic.
Delivering genetic material to cells of interest is one of the biggest challenges in developing a successful gene therapy. Genetic material injected directly into the bloodstream would be exposed to a number of enzymes which could degrade it before reaching target cells. Furthermore, genetic material cannot be easily inserted into a cell and requires a form of carrier called a vector. Think of it as a Trojan horse used to protect the cargo material from enzymes present in the blood and “trick” cells into taking up foreign genetic material. An ideal vector should be able to accommodate foreign genes of sufficient size, not trigger an immune response, and deliver gene to a specific cells.
Deactivated (“turned off”) viruses are commonly used as vectors in gene therapies. In this type of formulation, a viruses’ natural ability to infect cells is hijacked to deliver therapeutic genes. Unfortunately, viral vectors suffers from bad reputation as term virus is often associated with disease and mortality. Indeed, side effects of this vector can include an immune response, and for this reason research in the area of gene therapy has recently shifted towards the use of nanomaterial based delivery systems.
Nanomaterials are defined as materials with one or more external dimensions in the size range between 1 and 100 nanometres. For the past few decades there has been a rapid rise in the use of nanomaterials in a wide range of biomedical applications, with extensive research now focused on using this technology in gene therapy. We have witnessed effectiveness of nanomaterial based vectors during the global coronavirus disease 2019 (COVID-19) pandemic. Two vaccines (Spikevax® and Comirnaty®) used a type of nanomaterial called a liposome to deliver a single stranded genetic material, messenger RNA (mRNA) which codes for the production of antibodies against the spike protein on the coronavirus. Unlike some of the viral vectors, nanomaterial based delivery systems are considered biocompatible with a low risk of causing an immune response. Additionally, they can accommodate larger genes in comparison to viral vectors and can be relatively easily modified to target specific cells making them superior over traditional viral vectors. Figure 1 highlights some of the benefits of using nanomaterial based vectors in comparison to traditional viral vectors.
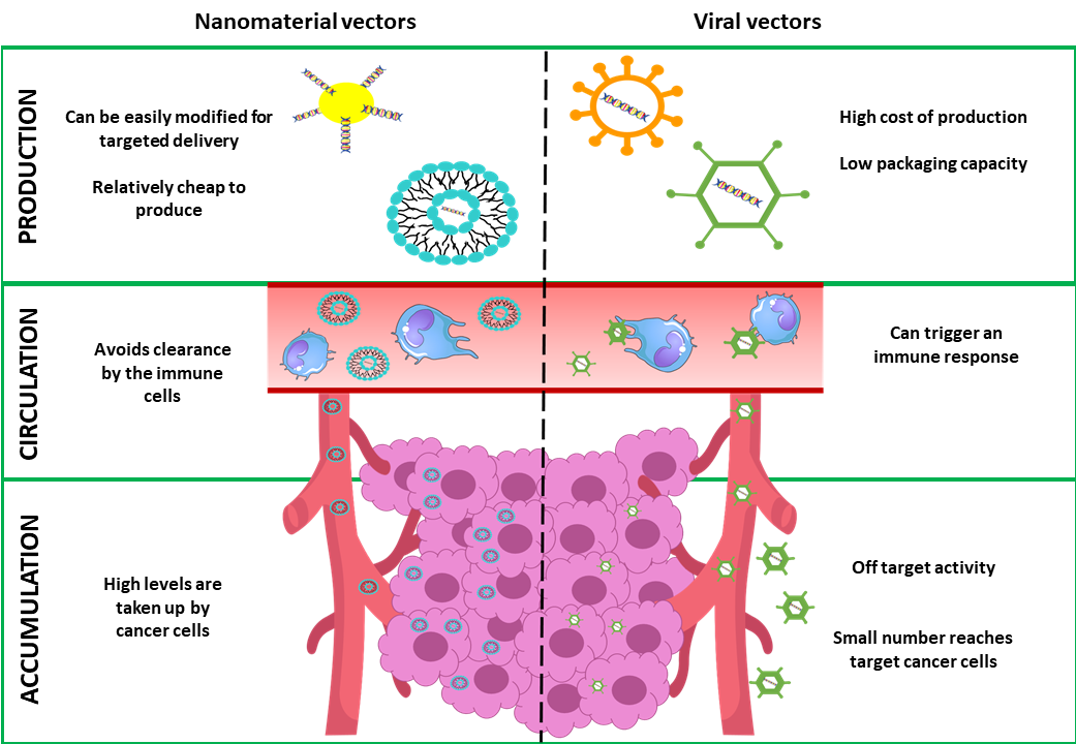
Figure 1: Comparison between nanomaterial based vectors and viral vectors for cancer gene therapy
Graphical representation of how nanomaterial based vectors (left hand side) overcome limitations of traditional viral vectors (right hand side) in cancer gene therapy. Image not to scale.
Our POI project led by Professor Tracy Robson, aims to harness the potential of nanomaterials in gene therapy for ovarian cancer. Over the past few years we have been investigating a protein called FK506-binding protein like (FKBPL). Our results indicate that this protein plays a role in inhibiting a number of processes occurring in cancer such as formation of new blood vessels (angiogenesis), tumour growth, and cancer cell migration, with the end result of slowing tumour growth. In order to increase the amount of FKBPL produced by ovarian cancer cells, we will deliver an artificial gene (transgene) coding for this protein using a peptide based nano-delivery system called RALA. This new technology allows us to deliver full FKBPL gene, something we could not achieve before. Although our research is still in its infancy, nanoparticle vectors have already solved one of the big delivery issues. We are excited to further investigate how this novel gene therapy will perform in treating ovarian cancer, with hope of bringing it to clinical trial.
Author: Dr. Anna Bogdanska
.jpg)
Author’s note:
After completing my undergraduate degree in Biomolecular sciences in Technological University Dublin, I interned at the department of Obstetrics and Gynaecology in Trinity College Dublin. I have recently completed my PhD in the Nanomedicine and Molecular imaging group in Trinity College Dublin, during which I had an opportunity to contribute to a number of pre-clinical studies of novel nanomaterials for medical applications. I am excited to work as a Postdoctoral Researcher on this project, under the supervision of Professor Tracy Robson. I hope that my personal interest and experience in areas of ovarian cancer and nanomedicine research will benefit the project with an ultimate goal of delivering a new personalised treatment to ovarian cancer patients.
My first experience in a cancer research laboratory
Written by: Zarghona Hassani, Apoptosis Research Centre, NUI Galway
Published: June 20th 2022
When I arrived in Ireland, I never thought that less than a year later, I would be an intern in a cancer research laboratory. I studied science at Kabul University, where I specialized in chemistry. After obtaining my degree in chemistry, I worked as a teacher and I became the principal of a private all girl school. I was responsible for more than 500 children and approximately 70 staff. Working as a principal of the school was not easy for a woman, especially for someone at my age. But, I was happy in my job, I enjoyed working with the students and staff, and welcomed the challenges it brought. I felt I was helping bring about change and doing something positive for the people in Afghanistan. I was happy with my life and career.
When the Taliban took control of Kabul in August 2021, my world was turned upside down. I feared for my safety, and I had to escape from Kabul. Eventually, I made it to Qatar where I waited for about a month. During this time, I didn’t know where I would end up. Thanks to the diplomatic efforts of two Irish women, and the charity Ascend, I obtained a visa to move to Ireland, as part of a group of 20. In November 2021, I boarded a plane to Dublin.
My first impressions were that Ireland was very calm. I wondered where all the people were! It was so different to where I grew up. Kabul was a very big, busy and lively city with a population of 7 million. The streets were always bustling with people and traffic; shops were always open. My new home was near a small town in county Galway with forests and many green fields. I knew it would take time for me to get used to this new environment. I had never left Afghanistan before, so everyone and everywhere in Ireland was so foreign to me. I had also left behind my family and my childhood friends, I missed them and I was lonely but I was determined to change this.
I was committed to starting my life and being happy in Ireland. I took English lessons and I worked in various jobs all within few months of arriving in Ireland. All this time, I missed the career that I had left behind in Afghanistan. Because of the new laws implemented by the Taliban, Afghan women no longer receive a formal education. This makes me sad as education is so important and has played such an important role in my life. In Ireland, I wanted to pursue a career in science and education. But with my qualifications, it would be difficult to get the job that I wanted. I knew that I needed to upskill to achieve this.
I started investigating how I would access the Irish education system. During this time, I met Professor Afshin Samali. He began to advise me on the various ways that I could access the Irish education system. Together, we explored various possibilities, and decided that if I pursue a master’s degree by research in the biomedical field, I would be in a stronger position to pursue my dream in Ireland. Completing a master’s degree in science would complement and increase my skills in the sciences. I would be able to gain practical hands-on experience of working in a research laboratory as well as broaden my understanding of cancer.

Zarghona Hassani in Prof. Afshin Samali's laboratory in the Apoptosis Research Centre in NUI Galway.
I was excited to be offered an internship by Precision Oncology Ireland and Apoptosis Research Centre at the NUI Galway to work with Prof. Samali. This meant I could experience working in a cancer biology research laboratory and learn some biomedical laboratory skills. It has been such a wonderful experience. During my internship, I have learned a lot. I have learned how to grow human cancer cells for experiments and to use many different types of equipment, all of which I had never seen before. Now I can culture cancer cells, I can count and plate cells from experiments, treat them with different anti-cancer drugs and determine outcome of drug treatment. I have also received training in laboratory health and safety, RNA isolation, reverse transcription, and PCR.

Zarghona (centre) with members of the lab during her internship
The internship was not easy because of the language. I knew general English and can easily have daily conversations. However, I didn’t know scientific English so there has been a sharp learning curve. I feel like I have had to study three times as much as others. Despite this, I gave weekly presentations to the group on a topic that is relevant to the work I was doing. For example, a question from one week was ‘does thapsigargin activate the unfolded protein response?’ To answer this, I designed and executed an experiment and analysed the data. I used the results of the experiment to make a presentation and explained my findings to the group.

Afshin Samali, Zarghona Hassani, Ciarán Ó hÓgartaigh (NUI Galway, President), Ciaran Cannon TD.
In the laboratory, people are from different countries around the world. They always help each other and they are so kind. One interesting thing to me is that they are always ready to have fun, they celebrate the small things as well as big achievements. I like this. I have enjoyed each day. I have met new people and I have learned new things. I felt welcomed and part of the team! I plan to enrol in a master’s degree by research in Biochemistry at NUI Galway and work under supervision of my mentor, Prof. Samali, to continue on cancer research. I have two immediate challenges ahead of me. One is that as a refugee I don’t have all my documents from Afghanistan to complete normal application route for university entry. Second, is finding the resources to fund my studies is going to a major hurdle. I know there is a lot more for me to learn, I know there will be many challenges ahead that I have to overcome, but, I am excited to get started and with teamwork I can get through one day at a time.
--
Zarghona Hassani was awarded an internship by Precision Oncology Ireland and the Apoptosis Research Centre at NUI Galway to work with Prof. Afshin Samali. We are proud to welcome her as part of the POI team.
June 20th each year is World Refugee Day, which celebrates the strength and courage of people who have been forced to flee their home country to escape conflict or persecution. You can find out more about the UNHCR here.
Ascend, the charity that helped Zarghona to come to Ireland, aims to empower young women and girls through the sport of mountain climbing. Find out more here.
What can a Lorry tell us about Tumour Immunology?
Written by Zak Kinsella
Published 21st July 2022
I have today the bittersweet pleasure of writing about my work to a wider audience. Sweet, because I have you - the readers - interested in the workings and wider research of myself, which is an honour and particularly cool if I do say so. Bitter, as my field’s writing style is known to be one of the most potent soporifics around, so I have to try and wrestle with that. Off to a good start, let’s see if I can bring you on the journey through my work. In a worst-case scenario, you have some content to hand that can put you to sleep no matter the circumstance!
To begin then, I’ll try to paint a picture. A tumour is much like a city. No, not in that way…but more so an environment of closely related processes, each working together, relying on the other, with traffic moving between its parts, a purpose in mind, to form a wider structure that we give a name. My research is focussed on deciphering the significance of these cityscapes and the purpose of particular neighbourhoods within – often called the ‘tumour microenvironment’. Many happenings occur in these neighbourhoods, and it is only relatively recently that research is beginning to peer into and unfold the meaning behind them. The example that I hope to explain today is one on the importance of traffic into and out of the breast cancer microenvironment. In particular, the immune system as traffic (Figure 1), and what we are trying to do in understanding how these metaphorical HGVs and their mileage affect the city; with hope that this information can be used in clinical practice to aid and support patient outcome decisions.
Figure 1. An example of a patient's breast cancer. Purple annotations show the border between invasive and non-invasive tumour area. The high volume of brown staining is indicative of large immune traffic through this tumour. Stained with CD45 (DAB, brown), counterstained with haematoxylin.
HGVs are curious things, though. On the one hand, their purpose is to supply behind the scenes the necessary bits and bobs for the effective continuance of the cities. On the other, they’re not very capable at doing this and instead are very effective roadblocks of the motorway systems. Likewise, in our bodies it is known that our immune system supplies a useful function, performed in the background unknown to us, by eradicating many cancers in their infancies. It only fails to do so when demand is highest, and the system is stressed continually. This causes what is known as ‘immune escape’, where be it by genetic alterations or other complex interactions, the tumour figures out a way of avoiding traffic jams, or else uses them to its advantage. The immune system is then rather like these HGVs, but we need to understand their effects.
In order to decode the traffic of the city, we need to know which HGVs are most useful to the city. But how do we label them - the useful and the not-so-much? Where are the HGVs going? Are there any ports or docks - areas particularly high/low in HGV density? What else could be key to HGV transport? To answer these questions, we must create a map of their positions and interactions.
According to an extensive 30 second Wikipedia search, a cartographer ‘…must typically collect, measure, and interpret data to create and update maps’. In the lab, we begin our mapping process by taking thinly sliced patient tumour tissue and staining it with a chemical dye specific to the HGV of interest. We add other dyes too, that allow us to collect a view of city architecture – the invasive tumour tissue and non-invasive tumour tissue. We then take satellite-like photographs at high resolution that allow us to see the city from a birds-eye view (Figure 2).
Figure 2. An example high-resolution image of ER+ Breast Cancer stained with the pan-immune marker CD45 (brown), counterstained with haematoxylin (blue). Excerpt shows a magnified region of the tumour at 3X magnification.
Traditionally, a trained professional would look at this map, in areas deemed representative of the city, and measure the labelled ‘HGV traffic’ there as a percentage. “This region is covered by 20% of HGVs”, for example. The average percentage of different regions is deemed the ‘score’ for the city. While undoubtedly this is useful, it is not a true reflection of the city’s HGV processes altogether. We must then employ a method that allows us the ability to see all HGV processes, all at once, all over the city. Only this way can we begin to pry out more crucial information on what our HGVs actually mean. To achieve this, we use machine learning and what is called ‘Digital Pathology’ on our labelled tumour tissues (Figure 3).
Figure 3. An example of one, trained digital pathology classifier to show location, number, percentage, and density of infiltrating immune cells. Imagine city architecture in red, roads in green, and our coveted HGVs in magenta. Tumour has been stained with CD45 (brown), counterstained with haematoxylin (blue). TIL = tumour infiltrating lymphocyte.
Besides providing pretty pictures, Digital Pathology has the potential to count every crucial piece of information we need, automatically. Like any brain, it first requires training on how to see and use information. Other colleagues and I in Precision Oncology Ireland collaborate with a pathologist – the trained professional – to ensure our training wheels are true and proper guides to the newfound brain. We then, with nervous apprehension, set it running without support and see how it performs. With enough training and guidance, the digital brain becomes quite adept. We continually test performance until we can be sure that falls or crashes, bumps and scars, are kept to a crucial minimum before pushing it off to cycle our birds-eye images for us (Oh, how quickly they grow!). In this way, we can be fairly certain that the results of the algorithm will be similar to our own, however, performed much more quickly and in much greater detail, as counts will be made in the entire image rather than in defined regions within.
Because our digital brain can count every lamppost, every building, every road, every HGV; we receive data that is fully quantitative. We then don’t have to infer how many HGVs there are on average – we now have a very precise number, a co-ordinate location, and a specific type of every HGV encountered in the city. This information can be used in a suite of tests to more accurately tell us the effects of certain HGV traffic, at certain times. Which HGVs are most useful, which not so much. Where HGVs are exactly, and what they may be interacting with (Figure 4).
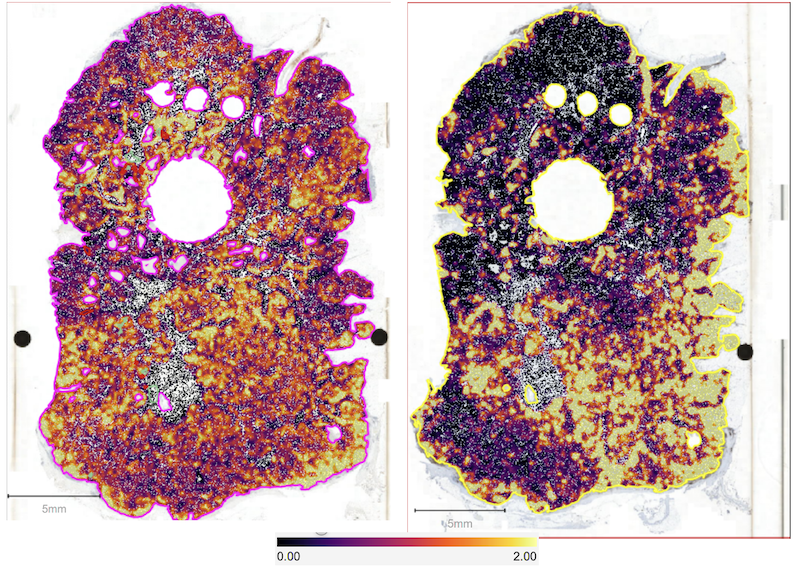
Figure 4. A heatmap of positive markers within two sections of breast cancer from the same patient. Marker density is shown by the key. The left image shows Ki67+ tumour cells - a marker of which tumour cells are actively growing. The right image shows CD45+, a marker of overall immune cells and their ‘neighbourhood’ clustering within the ‘city’ of this tumour.
What we are finding with this technology is that the presence of certain immune cells; their number; their location; whether they talk to each other on the road or avoid one another entirely, can predict how a patient’s breast cancer is likely to develop. The presence of high CD45 in these figures for example – a label of all HGVs as it were – we are beginning to show can predict worse survival chances in a subset of breast cancer patients [1].
Having at hand a map of tumour-immune interaction, with very high resolution, produced automatically, in bulk, rapidly and reproducibly, on every immune cell type in a tumour, is one of the next evolutions in the clinical decision-making process. There are many obstacles to overcome, but we hope and are actively working toward a way to facilitate this process, all to the benefit of breast cancer patients within Ireland and the community at large! Hopefully I have communicated an overview of my work effectively to you, in an interesting and digestible way. Perhaps also this will persuade you to look positively on your next traffic jam, whenever it occurs (it’s always good to end on a joke!).
--

Zak Kinsella is a PhD Student with Precision Oncology Ireland, based in Prof. Darran O'Connor's group in RCSI. His research aims to better understand the impact of immune cells on tumour progression, with a particular focus on breast cancer.
--
References
[1] https://doi.org/10.1200/JCO.2022.40.16_suppl.e12555.
How to be in the 3%: work, luck and persistence
My name is Matthieu Moncan, I’m a French postdoctoral researcher working with Prof. Adrienne Gorman in the Apoptosis Research Centre at University of Galway. In 2022, I joined Precision Oncology Ireland thanks to an MSCA - DevelopMed fellowship to investigate targeting the unfolded protein response for cancer treatment. My current position as an academic researcher is the result of 7 years of unexpected challenges and hard-won victories. These experiences validated what my lecturers used to tell new students: “3% of you will continue in research. 1% because they reach a high scientific level, 1% because they will be lucky and 1% because they will be persistent.” I’d like to share some of my story here to illustrate the truth in this.
Since middle school I have been fascinated by cell biology and genetics. Raised in a culture promoting equality, I was saddened to see the inequalities experienced by some people who live with genetic disorders. The idea that genes define traits that can’t be altered touched a nerve for me, especially if it meant that people would live their whole life with diseases deriving from bad luck in the genetic lottery. This inspired me to become a researcher in human genetics in order to help us to characterize, diagnose and treat genetic diseases.
I started on my path to achieve this goal by studying biology in Paris, specializing in genetics for my Masters. I completed three internships: one in the Cancer Research Centre, in Edinburgh, UK; one at St Jude Children’s Hospital in Memphis, USA; and one at the Imagine Institute, Paris, France. After graduation, I applied for PhD funding for a project on the genetic origins of autoimmune diseases. For the first time in my career, I encountered a major challenge – there was stiff competition for graduate research funding. My first attempt to earn a fellowship was unsuccessful. This first rejection was gut-wrenching to me since I was inexperienced and did not realise how commonly funding applications are rejected. However, the review panel did comment favourably on my project, which gave me confidence to not dwell on this failure. I soon made another application, this time a successful one to the Arthritis Foundation which awarded me funding to do a PhD.

Doing a PhD was both an incredible and a demanding experience. I thoroughly enjoyed the work, culture of knowledge sharing and the stimulating intellectual environment where I could share and discuss ideas. Apart from research, my PhD experience also gave me lots of transferable skills such as independence, management, critical analysis, communication and teamwork. Now when I scroll on professional social media networks, I see that many of my peers have chosen varied career paths, all based on a common love for science expressed in different ways: public or private researcher, scientific communicator, clinical project manager, medical writer, lecturer, sales representative, patent agent, scientific consultant, amongst others. All these paths have in common our love for science, and we are all happy to be able to express it every day in our own way.
With my PhD in hand I set about finding a postdoctoral position. This was even more challenging than finding a PhD position. After several unsuccessful applications I decided that I needed to change my strategy and to build a position for myself. To do this, I needed to have three things: a laboratory, a project and funding. I spent another couple of months trying to find a laboratory that would accept to support me, using the network I had built during my PhD. Here luck played a part. I met Profs Afshin Samali and Adrienne Gorman at a conference in Paris. We kept in touch and they agreed to help me. Despite their laboratory being specialized in cancer, we shared a common interest in IRE1α signalling. With their help, I designed a project in line with their research programme and the skills I developed during my PhD. But I still needed to secure funding.
I spent several months building a distant working relationship with them. During this period, I took opportunities Profs Samali and Gorman gave me to strengthen my CV, including publishing a review article and performing peer-reviews for a scientific journal. Meanwhile, I was unemployed in France. So, I took a position as a laboratory technician in the Compiègne-Noyon Hospital where I set up a new molecular biology platform to deal with the high demand for Covid19 testing. Also, around this time, I had two job opportunities either as a scientific consultant and as a scientific sales representative. However, my experience at the hospital helped me to understand that while my background was a perfect fit for them, in my heart I wanted to follow a career path in research. I longed to be in an environment with freedom to explore and be challenged in scientific debates. These job opportunities, which I rejected, nevertheless reinforced my determination to come back to a research laboratory.
Prof. Gorman and Prof. Samali informed me they had a vacancy for a research assistant. I decided to go for it and was successful. Later I learned that in addition to my basic qualifications, my expertise on the UPR and the perseverance I demonstrated during these two years of collaboration had been the deciding factor in my success.
A few weeks after the end of my contract at the hospital, I moved to Galway in the west of Ireland. It was great to be back working in a research lab, but I was acutely aware that it was only a 1-year position. I viewed it as an opportunity to get postdoctoral funding. Fortunately, all the work I performed during these two years improved my CV and this position opened new funding opportunities to which I applied. In August 2021 my application to the DevelopMed program was successful and in April 2022, exactly 3 years after I finished my PhD, I started my position as a postdoctoral researcher. Today, I’m working in a new research area. When I look at the paths that my graduated classmates took, during and after the PhD, I see that all of us faced various difficulties and challenges. Personally, I’m happy to have taken this journey to working as a researcher, despite the detours along the way. I hope this opportunity as a postdoctoral researcher will allow me to progress on a career path in line with my inspiration to help people suffering from genetic diseases and my love for biology.
Today, as I recall the words of my lecturers, I think that being part of only one of the three “1%” categories would not have been sufficient. The three qualities were necessary. I developed scientific skills which were recognized by my PhD and experienced researchers who wrote recommendation letters for me. I was persistent enough to seek and seize as many opportunities as possible. And this persistence encountered some luck, when I met people who gave me advice and supported me along the way.
The multiple possibilities of small microRNAs in breast cancer
Written by Dr. Vinitha Richard
While it may seem small, the ripple effect of small things is extraordinary.
Matt Bevin
The size of an early-stage primary breast tumour might range from 1mm-20mm, which could be related to the tip of a sharpened pencil (1mm) or the size of a peanut (20mm). Even a small tumour volume harbours millions of cancer cells, few of which have the ability to escape the tumour barrier even at an early stage and find their way to the bloodstream as metastatic cells. Early detection of the presence of tumours in the body increases the chances of disease-free survival.
Flooded with technological innovations and enhanced healthcare opportunities, we have surpassed several milestones in the efficient diagnosis and multiple treatment options in the fight against cancer. Over the years we have devised and implemented tactics to target the proteins and genes related to breast cancer, which is one of the easily detectable solid epithelial tumours. Yet we witness an increasing trend of high rates of breast cancer among women worldwide. This calls for readdressing the current scientific perspective of the disease and the mechanisms associated with the progression and recurrence of breast cancer in selective patients.
Just like the unique physical characteristics such as fingerprints, iris, and palm or finger vein patterns termed biometrics that can specifically recognize and identify an individual, we need a molecular biometric standard that can accurately detect the presence of cancer in an individual. Such an operational molecular biometric system should also provide information as to the response of the individual to a potential therapeutic regimen, the chances of their long-term survival, and also predict the risk of relapse after a period of disease-free status.
From the initial discovery of microRNAs in 1993 by Lee and Ambrose of Harvard University, USA, microRNAs have garnered immense attention as key players regulating gene expression right from simple roundworms to even more complex multicellular humans. Made up of a combination of four nucleotides [adenine (A), Uracil (U), Guanine (G), and cytosine(C)], microRNAs differ from the messenger RNAs (mRNAs) in the length of the chain of nucleotides (~19–24 nucleotides (nts) whereas mRNAs range from 400-1500 nts in length. MicroRNAs bind to the complementary target sequences in mRNA leading to mRNA destabilization, degradation, the resultant decrease in mRNA expression levels and thereby interfering with the final production of a functional protein product. With more than >35 000 miRNA sequences identified in >270 organisms, miRNAs play a role in crucial cell activities such as cellular signalling, metabolism, cell differentiation and proliferation, and apoptosis by controlling the expression of multiple genes and are abundant in cells.
MicroRNAs may function as either oncogenes or tumour suppressors under certain conditions. ‘Oncogenic miRNAs’, are upregulated in cells and they tend to inhibit the expression of potential tumour suppressive genes, while ‘tumour suppressive miRNAs’ are down-regulated causing the augmentation of downstream signal pathways or oncogenes responsible for the development of tumours. OncomiRs and tumour suppressor miRs may target the same or different mRNAs intracellularly (tissue) or extracellularly (in circulation). Different cancers may share common aberrant miRNAs, although cancer-specific miRNAs are the major population.
Breast cancer has been categorized into various subtypes and treatment decisions have been made based on the surface expression of hormone receptor proteins oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor (HER2). Post-treatment and over a period of disease-free survival, several cases report the emergence of resistant cancer cells of a different subtype or receptor phenotype. This receptor discordance is attributed to the sudden loss of gain of receptor expression mediated by regulation of the receptor gene expression. MicroRNAs can modify the expression of genes post-transcriptionally and lead to the development of intratumoural heterogeneity.
The expression profile of proteins has categorized breast cancer into three major subtypes, whereas gene expression profiling has identified 5 major groups. Further a step, an exclusive microRNA profiling of breast tumour tissue led to the identification of 10 combinations of breast tumour subtypes and this widens the angle for implementing targeted treatment or precision medicine in breast cancer. Defining each of the subtypes of breast tumours accurately will lead to the identification of the tumour cells that will succumb to treatment and those that will resist. This will enable the prediction of patients likely to respond to chemotherapy and those who will not, thus sparing them with unwanted side effects of chemo. Also elucidating the significance of the identified microRNAs will pave way for utilizing them as therapeutic targets to alter the disease status of an individual. Though they are small non-coding molecules, the ripple they create travels from cell to cell bringing about significant changes in the human body that is yet to be disclosed through research.

Dr. Vinitha Richard is a postdoctoral fellow working at the University of Galway under Prof. Michael Kerin, as part of POI work package 1.4. "MicroRNAs For The Detection Of Breast Cancer"
How I got obsessed with PPI activities
The first time I came to hear about the term “Public and Patient Involvement” or “PPI” for short was in 2016. Professor Amanda McCann of UCD spearheaded the Patient Voice in Cancer Research (PVCR) initiative which aimed to actively engage those touched by cancer. Under this program, cancer patients, cancer researchers and others (patient advocates, families, carers, healthcare professionals and policy-makers) could get involved in discussions and decision-making processes that positively impact the cancer research landscape and outcomes for patients. In September of that year, my boss, Professor William Gallagher, asked me to show our lab to a group of patient representatives. As a former clinician turned full-time researcher, I had no idea that patients could be involved in laboratory research. We used to use patient’s samples given to us by our clinical collaborators, but that was where the connection stopped.
As part of this PVCR initiative, a group of cancer survivors came to visit our lab at Conway institute, UCD. During this visit, patients and patient advocates were shown what happens with the samples that they or their relatives have donated for research and how we use these materials for our day-to-day experiments. Very nonchalantly I agreed to take up this task, as I never say no to my boss! Four women visited our lab, some of whom had recently undergone chemotherapy and were donning scarves on their heads, still visibly living with some of the after-effects of their treatment. My heart sank as I described the experiment I was going to perform on that day with that tissue sample, how researchers use tissue samples to discover cancer biomarkers. The feeling that this piece of tissue might have come from one of these women standing in front of me hit me hard. At that moment, I realized I had never treated a tissue sample as if it had come from an individual (I might as well be facing one of them). Doing laboratory-based research made me so out of touch with patients that my sole thinking until then was my own personal development, which had nothing to do with patients. I was not aware of my responsibilities as a researcher, I had no sense of gratitude to the patients for the tissue sample that they graciously donated to us to help us better understand cancer. At that time, I was dreaming of a permanent job in academia, showing off scientific findings at international conferences, and climbing the career ladder. The sense that I was doing something that could impact someone’s life was not in my mind until then.

Looking forward from my first experience, I have not missed an opportunity to attend a PPI event. My experiences to date have included explaining science in simple terms to audiences outside the scientific community, showing visitors how a lab works, and making people aware of cancer and cancer research. Over the course of several years working with UCD's PPI program, I noticed that there are no patient representatives from ethnic minorities in any of the programs I attended. I thought there must be patients from ethnic minority communities, why they are not in the program?
Since I myself am a member of an ethnic minority group, I realized that not only are these patients not among the cancer patients' representative groups that participate in outreach programs, but a large portion of our community may be misinformed about cancer due to significant barriers and misinformation. Understanding cancer within ethnic minority populations is a multifaceted issue that requires careful consideration. Cultural beliefs, language barriers, and limited access to healthcare services often shape their perception and comprehension of this complex disease. Ethnic minority communities may have unique cultural interpretations of cancer, including spiritual or traditional beliefs that influence their understanding and treatment-seeking behaviours. Moreover, language barriers can impede effective communication between healthcare providers and patients, hindering the dissemination of vital information about cancer prevention, early detection, and available treatment options. In addition, socioeconomic disparities and limited access to healthcare services further compound the challenges faced by ethnic minority populations in obtaining timely and appropriate cancer care. To address these disparities, it is essential to develop culturally sensitive education campaigns, increase language support services, and improve access to affordable and quality healthcare resources, ensuring that all individuals, regardless of their ethnic background, can navigate the complexities of cancer with informed understanding and support.
An opportunity arose in 2020, due to my ethnic and religious background I had connections to both Bangladeshi and Arab communities here in Ireland. Through Precision Oncology Ireland (POI), supported by SFI, we co-developed a programme of events with two minority ethnic community groups in Ireland: a Bangladeshi community group, and an Arabic Muslim community group. We named the program ‘Invisible Spectrum’. Ethnic minority groups can be ‘invisible’ in the cancer research landscape - most research studies in the field of cancer and genetics have been focused on white Caucasian populations, and minority groups such as Southeast Asians are significantly under-represented. Invisible Spectrum engages with minority communities with the goal of informing, inspiring and empowering community members to become active participants in the national conversation on research and science. At these community-focused multi-lingual event, members of ethnic minority community groups met scientists working at the cutting-edge of cancer research in Ireland. Participants discovered how doctors decide what treatments to give to cancer patients, and how cancer research helps them to navigate this decision. Participants also learned about cancer screening and how to be aware of cancer symptoms, with a translator and an Irish Cancer Society nurse on hand to respond to any queries or concerns.
Before the event, we engaged with the key social leaders of these communities to discuss the format of the events. Our events were meticulously planned to make sure it meets the expectations of the participants. In 2020, during our first program, we aimed to reach out to the Bangladeshi and Arab Muslim community, especially to the elderly female members. Many of our female community members practises Purdah. The Purdah practice is a traditional cultural and religious practice observed by some Muslim women to maintain modesty and privacy in the presence of unrelated men. While the practice varies across different Muslim communities and regions, it is generally a personal choice made by women to uphold their religious and cultural beliefs. Due to this Purdah practice, maybe many of these female community members feel uncomfortable to disclose their early signs and symptoms of many diseases including cancer to a male GP. They might face language barriers also in explaining their health issues appropriately.
We aimed to address these issues in our 2020 program. All talks were translated to Arabic and Bengali; we organised female only session, presented, and participated only by the females. In the feed back notes, they praised the event and wanted more of this from us!
For 2021, Invisible Spectrum 2.0, was built upon the success of the previous event that aimed to produce a deeper engagement with the ethnic minority community of Bangladeshis in Ireland. This time, we aimed to re-engage the members of this community on the topic of cancer genetics (including references to DNA, cells, and cancer) under the banner “Invisible Spectrum-My DNA, My Health”. The ultimate ambition for Invisible Spectrum 2.0 was to develop a template for engaging minority communities with complex research topics such as genetics of cancer. Dr. Terri McVeigh, a consult clinical geneticist from Royal Marsden hospital explained risk of genetic diseases specially cancer in such simpler terms where I translated every word of hers to Bengali for the community members. During this program, eminent cancer researcher such as Prof. Walter Kolch, Prof. William Gallagher also spoke about genetics in cancer, their talks were also dubbed in Bengali so that everyone in the audience understand what was being discussed. The feedback from our attendees was great, this was a successful program again!

After two consecutive online successful programs engaging with minority community, we delivered our 2022 program face to face. We had a fantastic organising team; the minute detail of the event was scripted, and we all did our parts accordingly. We invited around 100 Bangladeshi community members to Invisible Spectrum 3. The event was held on Saturday, 19th November. Our 3rd annual event, this was the first time that we could hold on in-person event thanks to easing of Covid restrictions and financial support from Science Foundation Ireland. Featuring talks from researchers, separate breakout sessions for men and women, networking and discussion opportunities and even a cell biology focused art workshop for children, the day was a huge success.
Engaging with ethnic minority groups in Ireland for cancer awareness program can be challenging at times. As an organiser, besides being respectful to cultural sensitivities, one has to know how to approach the community members for such an event and how can one reach out to a wider targeted community groups by engaging with key social leaders. Moreover, we constantly worked on new ideas how best we can target key groups within these communities. We knew we wanted to have the opportunity to reach all members of the family, and to do so all had to be catered for. In order to make sure everyone had the same chance to attend the event, a dedicated children’s room was set up with activities so that Mums and Dads could attend our talks freely. These young families often would not have the luxury drop the kids at grandmas before coming to the event on Saturday, so we provided supervised child care for two hours with drawing/painting session in a nearby room, while the parents attended gender specific cancer awareness sessions.

Cancer awareness programs play a crucial role in ethnic minority communities, highlighting the importance of early detection, prevention, and access to healthcare services. These communities often face unique challenges when it comes to cancer, including language barriers, cultural stigmas, and limited knowledge about available resources. By raising awareness, these programs can empower individuals within these communities to recognize the signs and symptoms of cancer, understand the significance of regular screenings, and make informed decisions about their health. Moreover, they foster a sense of solidarity and support, breaking down cultural taboos surrounding cancer and encouraging open conversations about the disease. Ultimately, cancer awareness programs among ethnic minority communities can save lives, reduce health disparities, and promote equality in cancer prevention and treatment.
Cancer research is not typically a patient-facing discipline, but in the new era of precision medicine, it is more important than ever to involve patients in our research and gain their perspective on the challenges we face.

About the Author: Dr Arman Rahman is an assistant professor at UCD School of Medicine and Translational Research and Engagement Manager for POI. He manages the POI Tissue Imaging Platform as part of work package 4.4
"Why are you doing a PhD?"
I am often asked by friends and family “why are you doing a PhD?” Why, after finishing a masters, have I decided to go back and dedicate another 4 years to research and study? For me, it has been clear from a young age that I have always had a love for science and the natural world, and since the age of 8 I have always told people that I wanted to be a scientist when I grew up. Initially fueled by my love for astronomy based off of Sir Patrick More's "The Sky at Night" and programmes featuring Professor Brian Cox on BBC, I decided that I wanted to study physics. Unfortunately, my time in secondary school taught me that I was not very good at physics. However, I was fortunate enough to have a genuinely exceptional biology teacher, and it was in his class that I began to foster a love for biology.
Of all the different sections of biology that were learnt, I saw myself being drawn towards genetics the most. From the get go, I appreciated the predictability it offered. How through the study of genetics, we might one day be able to accurately predict any ailments a person may suffer throughout their life and plan accordingly (Although, my undergraduate in Genetics has taught me that things are far from that simple). The second aspect of genetics that resonated with me was the fact that it is still an emerging field on the frontier of science. The fact that the whole field of molecular biology and genetic engineering is in a golden age in terms of research and applications played a large role in guiding me down this career path.
However, what ultimately made me decide to dedicate myself to cancer research was my own sister being diagnosed with leukaemia in early 2019. I’ll start off by saying that this story has a happy ending! Thanks to the spectacular work of everyone at Crumlin Children’s Hospital she has been cancer free for almost 3 years, and is now completing her undergraduate degree in nursing.
Naturally, my sister’s battle with cancer has had a huge impact on me and what I want to do with my career. My sister was fortunate enough to be treated with a novel immunotherapy, and when I realised just how recently developed the treatment was (it was only approved in the EU 6 months before she was diagnosed!), it really made me appreciate all the groundbreaking advancements oncology has been making. I realised that without people who dedicate their careers to researching new treatments for cancer, that my sisters treatment wouldn’t have been the success story it is. Due to my sister’s experience, as well with my interest in genetics, I decided that I wanted to explore new avenues that would lead to more cancer treatments that are more tailored to each individual. This would lead me to joining Precision Oncology Ireland as a PhD student in 2022.
Another aspect of POI that my sister’s time in hospital made me appreciate was the importance of patient outreach. Due to having some experience with cancer from classes I had taken during my undergrad, I was better able to explain my sister’s condition to both my parents and extended family. I realised that I quite liked explaining the science behind cancer to people from non-cancer related backgrounds, and it’s always great to show the general public what we get up to in the lab through outreach events and tours.
And a final element for why I chose to do my PhD with POI is because of its cooperation with charities. My family benefited immensely form the support of charity during my sister’s time in hospital. Due to us living in Kerry, it wouldn’t have been feasible for my parents to commute to Dublin while my sister was in hospital, and Dublin is well known for its lack of accommodation. Initially, my parents would take turns sleeping in the hospital. Fortunately, the Ronald McDonald House charity stepped in and offered us a family room. Having a place in Dublin that we could all stay in made it significantly easier to be there for my sister. Additionally, we were lucky enough to be approached by the Make-A-Wish foundation. Despite being held back due to the COVID-19 pandemic, Make-A-Wish fulfilled my sister’s wish and send the whole family to Center Parcs back in May. It is hard for me to properly put into words just how critical support from charities is for individuals undergoing cancer treatment and their families.
So, when asked why I decided to pursue a career in cancer research, I always think back to my families experience during my sister’s diagnosis. Aside from cancer research being a good match for my background in genetics, I wanted to do a PhD with POI as a way to give back to the organisations and charities which I have so benefited from, and to make a difference in the fight against cancer.
-400x400-204x204.jpg)
About the author: Brandon Sugrue is a PhD researcher in Prof. Róisín Dwyer's group in University of Galway. You can read more about Brandon's project here
What do bacteria have to do with cancer?
Human beings are a symbiont of human and microbial cells, with more bacterial cells in our body than human cells (around 2:1). Although most of these microbes live within our gut, collections of microbes are also found in most body parts. These collections of microbes within a given region are known as a microbiota, a term originally coined in 1988, and the collection of microorganism’s (microbiota) genes is known as the microbiome. These congregations of microbes, which may include not only bacteria but also fungi, yeast, and viruses, are site-specific, with the microbiota of each body part having distinctive characteristics regarding overall numbers and diversity of microbial species. The highest diversity (widest range of types) is found within the gut microbiome (GM) and generally speaking, the greater the diversity and number of bacterial species, the better for our health. Our GM represent a virtual organ that performs essential body functions such as digestion and metabolism of nutrients, maintenance of a healthy gut wall and defending against disease-causing microbes. Also, the GM is partly responsible for the development of a healthy immune system and brain. Even our moods are constantly being influenced by these invisible creatures who evolved hundreds of millions of years before us. However, with the evolution of the first gut in some ancestral worm, these single celled organisms have since evolved in step with their multicellular symbionts.
For humans, the first major colonisation event with our microbial partners is at birth, which depending on the mode of birth, will at first closely resemble either the skin or gut microbiota of the mother. After this, our GM is shaped by external factors such as diet, lifestyle, and environmental biodiversity, and as such, our microbiome composition varies with age. On average, the adult GM is composed of approximately 300-500 species. With age there is a decline in microbial diversity and by > 65 years there is usually a significant reduction in diversity.
 Dysbiosis (from Ancient Greek (dus- “bad”) and (bíōsis- “way of living”)) refers to an imbalance in our microbiome. Dysbiosis is an imbalance in the microbial diversity of the microbiome with a reduction in the number of beneficial species and an increase in the proportions of disease causing microbes. Dysbiosis negatively affects all the functions that our GM carries out, and if not brought back into balance, will lead to disease over time. Dysbiosis has been found to promote cancer growth, mainly through its interactions with the immune system, where it has been found to suppress the regular activity of the immune system to destroy any malignant growths.
Dysbiosis (from Ancient Greek (dus- “bad”) and (bíōsis- “way of living”)) refers to an imbalance in our microbiome. Dysbiosis is an imbalance in the microbial diversity of the microbiome with a reduction in the number of beneficial species and an increase in the proportions of disease causing microbes. Dysbiosis negatively affects all the functions that our GM carries out, and if not brought back into balance, will lead to disease over time. Dysbiosis has been found to promote cancer growth, mainly through its interactions with the immune system, where it has been found to suppress the regular activity of the immune system to destroy any malignant growths.
In a balanced state, the microbiome promotes positive health and prevents tumour growth by positively influencing the immune system and maintaining gut barrier integrity. Conversely, during dysbiosis, there can be a loss of gut barrier integrity, which can lead to potentially harmful bacteria making their way past the gut wall and taking up residence in other body compartments. Context is all important; a beneficial species that usually resides in the gut can have adverse effects in other areas of the body. This can result in chronic inflammation in distal sites and the production of cancer promoting bacterial by-products such as toxins which can cause DNA damage.
Chronic inflammation contributes to cancer growth at all stages, from initiation to progression and spread (metastasis). It has been found that up to 20% of all cancers are preceded by chronic inflammation at the cancer site. While in some cases bacterial species such as Helicobacter pylori, are directly responsible for some gastric cancers, most of the time bacteria are merely enablers of some aspect of cancer growth.
It was not until the advent of next-generation DNA sequencing that the influence of bacteria began to be teased out. Many of the residents of our gut cannot be grown in the laboratory, but now that we can read their DNA, they can be readily identified. This has led to the use of microbiome-wide association studies whereby all of the gut microbes can be identified from an individual. When this method is applied to populations with large sample sizes, it can be extremely powerful in allowing scientists to discriminate between beneficial and detrimental species for any disease, including cancer.
Very recently, this has been applied to the study of cancer treatments, where it has been found that the GM affects many types of treatment outcomes from chemotherapy to radiotherapy and the latest immunotherapies. Currently, many clinical trials are ongoing where faecal matter transplants (FMT) are being carried from patients who have had a good treatment outcome to patients who are missing these collections of microbes. This is equivalent to an organ transplant but is a black box approach, in that whilst we currently might not completely understand mechanistically what is going on, it can drastically improve the lives of individual patients.
Of the many beneficial molecules that bacteria produce, of which there are too many to discuss in a short blog, short chain fatty acids have been shown to have the most potent anti-cancer properties, with butyrate having been found to have the strongest effect. Members of the Firmicutes produce butyrate in the large intestine through anaerobic fermentation of indigestible dietary fibre and resistant starch. Butyrate’s protective properties, including anti-inflammatory effects, suppression of blood supply to tumours, and reversion of the silencing of tumour suppressor genes, among others. A high fibre diet promotes the maintenance of butyrate-producing bacteria, making it cancer-protective. Conversely, the opposite effect is true; depletion of butyrate-producing bacteria may promote inflammation and tumorigenesis systemically. If I have one bit of advice from my studies of the GM on avoiding cancer development, it would be to eat more fibre!

About the author: Ciarán Devoy is a PhD researcher in Prof. Mark Tangney's group in University College Cork. You can read more about Ciarán's project here .
The Trinity St James’s Barrett’s Oesophagus Biobank
My name is Cian Gargan and I am the Barrett’s oesophagus biobank manager in the Trinity Translational Medicine Institute (TTMI). I started my position here in April 2023 and my role is to find and recruit suitable patients with Barrett’s oesophagus in St James’s Hospital to our research program. After completing my undergraduate degree in biochemistry, I decided that I wanted to pursue a career in research but with a clinical perspective. I really enjoy my job as it allows me to meet patients, work as part of a clinical team and be involved with lab-based research. It is rewarding to know that as I recruit more patients to our research program, more research can be carried out that will help us better understand Barrett’s and its possible progression to oesophageal cancer. This may lead to discoveries that will allow for the development of new treatments for Barrett’s in the future.
 Barrett’s oesophagus is a disorder of the lower oesophagus where cells become abnormal and begin to behave like cells which are normally found in the intestines. This is known as intestinal metaplasia and is primarily caused by acid reflux affecting these cells over a long period of time. Symptoms of Barrett’s oesophagus include frequent and longstanding heartburn, difficulty swallowing, and a sensation of food stuck in your oesophagus. Barrett’s is usually diagnosed through an endoscopy (camera test) where samples of the oesophagus are taken and then later analysed in a laboratory. Individuals suffering from Barrett’s oesophagus can usually have their symptoms managed using medication prescribed by their doctor. Most people with Barrett’s oesophagus will not go on to develop oesophageal cancer, as the risk of progression is less than 1% per year but they are still recommended to have routine camera tests every few years. This is to check for any changes in the Barrett’s tissue that indicate oesophageal cancer is likely to develop.
Barrett’s oesophagus is a disorder of the lower oesophagus where cells become abnormal and begin to behave like cells which are normally found in the intestines. This is known as intestinal metaplasia and is primarily caused by acid reflux affecting these cells over a long period of time. Symptoms of Barrett’s oesophagus include frequent and longstanding heartburn, difficulty swallowing, and a sensation of food stuck in your oesophagus. Barrett’s is usually diagnosed through an endoscopy (camera test) where samples of the oesophagus are taken and then later analysed in a laboratory. Individuals suffering from Barrett’s oesophagus can usually have their symptoms managed using medication prescribed by their doctor. Most people with Barrett’s oesophagus will not go on to develop oesophageal cancer, as the risk of progression is less than 1% per year but they are still recommended to have routine camera tests every few years. This is to check for any changes in the Barrett’s tissue that indicate oesophageal cancer is likely to develop.
A biobank is a large collection of biological samples and healthcare information. The samples donated by patients in our biobank are stored in ultra-low temperature -80˚C freezers to be used by researchers at a later date. The Trinity St James’s Barrett’s oesophagus biobank is now over 10 years old and the largest Barrett’s biobank in Ireland which contains thousands of patient samples used in translational research. I recruit eligible patients with medically confirmed Barrett’s in St James’s Hospital who are booked in during their routine surveillance camera test. On the day of their visit, I meet each eligible patient, explain to them our research program, and give them the option to donate two small vials of blood, as well as biopsies taken from their Barrett’s tissue.
The donation of blood and tissue biopsies is completely optional and each patient has the right to change their mind at a later date. If that is the case, the bloods and tissue samples will be removed from the biobank and discarded. Each patient will also have their samples anonymised to the researchers to ensure their privacy.
If a patient is happy to take part, a note will be made on their chart, the nurses will take blood samples and the endoscopist will take additional biopsies for research. These samples will then be transported to the Trinity Translational Medicine Institute (TTMI). The research biopsies will either be used straight away by our researchers or I will store them in the biobank along with the processed blood samples for future use. The Barrett’s biobank is an important tool for researchers at Trinity College Dublin. As it grows we have more and more samples at our disposal to facilitate Barrett’s research. Multiple patients have donated several samples over many years which allows researchers to compare these samples to understand the development of Barrett’s over time. Since its inception, many discoveries have been made and published using samples from the Barrett’s biobank and multiple research projects are currently ongoing.

About the Author: Cian Gargan is the Barrett’s oesophagus biobank manager in the Trinity Translational Medicine Institute and St James’s Hospital.
Travel broadens the mind
My name is Donagh Egan, and I am a PhD student in Systems Biology Ireland. My research focuses on understanding how tumors evade the immune system, and exploring the use of drugs to enhance the immune system's ability to combat cancer. Recently, I had the opportunity to conduct research at The Francis Crick Institute in London, England, supported by an EACR Travel Fellowship and funding from my principal investigator. In this blog post, I wanted share my experience to highlight the importance of providing early career researchers with the opportunity and financial support to undertake research placements in different labs with diverse research backgrounds.
I applied for the EACR Travel Fellowship to collaborate with researchers whose expertise would complement my own. Engaging with researchers from diverse fields provides fresh perspectives that can guide new methods and insights for addressing research questions. Samra Turajlic’s lab at The Francis Crick Institute offered an ideal environment for this purpose. However, the high costs associated with opportunities in London can be prohibitive for early-stage researchers. The funding I received eliminated this barrier, enabling me to cultivate scientific collaborations and establish networks with esteemed scientists.
During my placement in Samra Turajlic’s lab, I collaborated closely with Dr. Irene Lobon, a postdoctoral fellow in Samra Turjalic’s group. Irene’s research is different to my own, focusing on how cancer evolves under the selective pressure of the immune system. Irene's expertise in this area provided a unique perspective to my own research questions, leading to answers and findings that neither of us could have resolved working independently. I also worked closely with a medical oncologist, Ben Shum. A key aspect of my analyses was understanding the clinical characteristics of patient data. Ben ensured that the data was correctly interpreted, which provided confidence that my own findings were grounded in correct assumptions.
The visit granted me access to novel data and the expertise to navigate it using innovative bioinformatic approaches. In doing so, I greatly improved my computational skills, helping me to uncover novel insights that will benefit my research going forward. Joining a new laboratory required learning and adapting to new processes and methods of communicating. As a results, my communication skills have seen benefited, and I now feel more at ease expressing my ideas and incorporating feedback into my research. I hold these skills in high regard, and recognize their applicability to my future professional endeavors.
Overall, I have gained invaluable skills that are essential for scientific research, which will undoubtedly elevate the quality of my work moving forward. This experience has opened my eyes to the importance of providing researchers with similar opportunities to advance scientific discoveries that will ultimately translate into better treatment options for patients in the clinic.

About the Author: Donagh Egan is a PhD researcher under supervision of Prof. Walter Kolch and Prof. Donal Brennan. You can read more about Donagh's research here
