Cancer and Stress: Targeting stress responses in cancer cells
We think of cancer cells as fast-growing, highly adaptable cells that can withstand and sometimes even thrive in extreme conditions. When tumours grow fast, the blood vessels that supply oxygen and nutrients to the tumour can’t keep up.
This can be a stressful scenario for a cell. Despite these stress-inducing scenarios, tumour cells continue to grow. Similarly, when tumour cells are treated with traditional therapies, like chemotherapies, these drugs should kill cancer cells but many cancer cell types display excellent abilities to avoid drug-induced cell death. One may even say cancer cells have developed a tremendous ability to cope with stress. So how do they do it?
Since joining the Precision Oncology Ireland team, my research has focused on one of these potential pro-survival mechanisms. We study a protein called Inositol-requiring enzyme 1 (IRE1) and we are particularly interested in how this protein functions to promote cancer cell survival, phenotype, and oncogenic behaviour. IRE1 is located in the endoplasmic reticulum (ER), a vast cellular organelle that assists in the processing of newly made proteins.
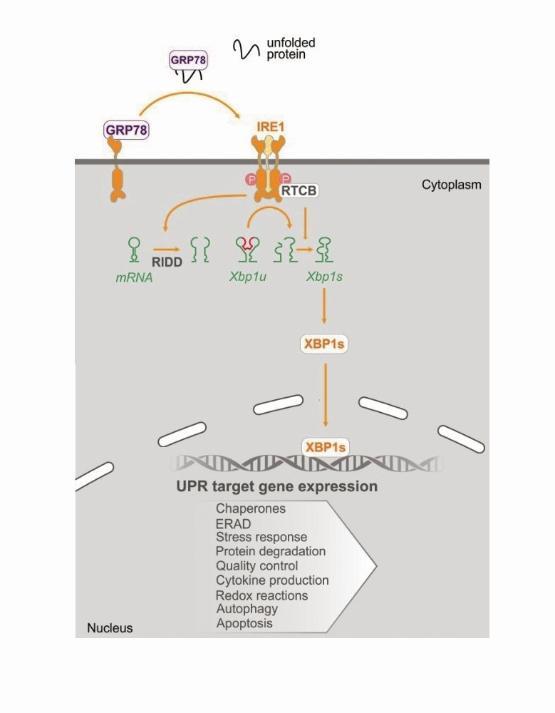
The proteins in a cell are made from messenger RNA (mRNA). The ER ensures that cellular proteins are made properly and have the correct structure necessary for them to perform their designated function/s. If a cell becomes stressed, e.g., if nutrients or oxygen supplies are low, newly synthesised proteins can often be made incorrectly. IRE1 senses these badly made proteins as they accumulate in the ER and it initiates a signaling pathway that acts to restore protein balance.
In its simplest terms, I like to think of IRE1 as behaving like a Pacman when it’s active. Active IRE1 acts to degrade mRNAs by breaking them up, much like a Pacman would if it continued to open and close its mouth and mRNAs were its dinner. IRE1 is a bit more specific about what it eats for dinner, but hopefully, you get the general idea! By degrading specific mRNAs, IRE1 acts to decrease protein burden in a cell. Additionally, one of the best-described functions of IRE1 is its cleaving of XBP1 mRNA. When 26bp of XBP1 mRNA is cut out, the two pieces of RNA that are left behind are re-ligated and this mRNA is translated into a highly active transcription factor called XBP1s. XBP1s acts to switch on genes that help to restore protein balance.
In healthy cells, the IRE1 signalling pathway is necessary for the cell to adapt to, or overcome, a stressful scenario. However, we think that cancer cells often utilise this same pathway to ensure that they can survive any stress they may encounter. Our group has previously shown that in multiple cancers IRE1 and its downstream signalling pathway is active and when some of these cancer cells are treated with certain chemotherapies, subpopulations of cells may capitalise on the IRE1 pathway to evade drug-induced cell death.
These observations have encouraged us to investigate the benefits of targeting IRE1 in cancer, by inhibiting its ability to degrade mRNAs (effectively shutting down its Pacman-like behaviour). Our research currently investigates in more detail the roles of active IRE1 in cancer cells as well as the therapeutic benefits of targeting IRE1 in cellular models of triple-negative breast cancer and pancreatic cancer. We believe that modulating IRE1 activity may be advantageous in the successful treatment of these cancers.
Author: Dr. Claire Robinson

Dr. Claire Robinson received a B.Sc and Ph.D. from University College Dublin, Ireland. She completed a Post Doctoral Fellowship at the University of Toronto, Canada. Claire currently works at the Apoptosis Research Centre in NUI Galway.

