

P2.7: Improving immuno-checkpoint inhibitor (ICI) therapies
Plain Language Statement
Ten years ago we first heard about a new type of drug that could actually help cure cancer. These drugs, called immune checkpoint inhibitors, were shown to be very effective in treating certain cancers.
To understand how they work, we need to talk a bit about their targets, proteins called immune checkpoints that can be found on cancer cells and immune cells. The role of these checkpoints is to stop the immune cells from producing a too strong immune response that could be harmful. Normally, these checkpoints are expressed on immune cells so that they can prevent their activation overshooting. In cancer, however, checkpoints are like Superman’s kryptonite. Cancer cells express molecules that engage the checkpoints and thereby actually can prevent immune cells from detecting and killing tumour cells. Immune checkpoint inhibitors can shield the kryptonite and allow the immune system to attack cancer.
These drugs work really well for some cancers, such as melanoma and lung cancers. It was all very exciting until we found that immune checkpoint inhibitors weren’t effective in all patients and they just didn’t work for some cancers like ovarian cancer.
We just don’t know enough about how these drugs work and how this kryptonite paralyses immune cells. That is why we teamed up with researchers and clinicians from Systems Biology Ireland and AstraZeneca. We want to learn more.
We started by mapping what is happening within an immune cell when a checkpoint is active and then what specifically changes when we block it with the drug. This allow us to identify key markers involved in the immune cell response to the blockade of a checkpoint. We can then evaluate these markers in immune cells of ovarian cancer patients to predict how each patient will respond to a specific checkpoint inhibitor.
This is important work because once we understand the behaviour of immune cells in response to the different immune checkpoint inhibitors, we could find the right combinations of drugs that would help improve treatment efficacy for patients.
Project Overview
Immunotherapy is a rapidly expanding area of research, that focuses on harnessing the power of the immune system to treat cancer. Tumour cells and the tumour microenvironment have the ability to suppress the activation of immune cells. This can be achieved by engaging inhibitory receptors, such as PD1 (Programmed Cell Death 1) and CTLA4 (Cytotoxic T-Lymphocyte Associated Protein 4) on T-cells. A type of drug known as an Immune Checkpoint Inhibitor (ICI) can block these interactions, relieve T-cell suppression and stimulate immune function. These drugs have shown promising success in several tumour types, most notably melanoma, lung and kidney cancers.
Enlisting the immune system to fight cancer is an attractive concept, as it promises to be effective against many different types of cancer. Unfortunately, responses are highly variable, and remain low and unpredictable in many tumour types. Moreover, toxicity due to adverse immune related events can be challenging. Nevertheless, enlisting the immune system to fight cancer is an attractive concept, as it promises to be effective against many different types of cancer. In addition, when patients do respond to ICI’s, the responses tend to be durable and long lasting, asserting that the immune system can successfully eradicate or control cancers which were previously deemed incurable. Thus, ICIs are exciting therapeutic agents, however an improved and deeper mechanistic understanding of their molecular mode of action could lead to wider application and novel combinations with other drugs.
A focus on immunotherapies is a strategic priority for both SBI and the industry partner on this project, AstraZeneca (AZ), and research in this area will be of global impact. Therefore, computational scientists, biologists and clinicians from SBI and AZ have teamed up to analyse the molecular mechanisms of action of ICIs and use this knowledge to improve the development and deployment of ICIs. We are focusing on ovarian cancer as a poor prognosis cancer that should respond well to ICIs. To date response rates have been poor and large clinical trials have failed to demonstrate efficacy so we expect decisive improvements to be possible.
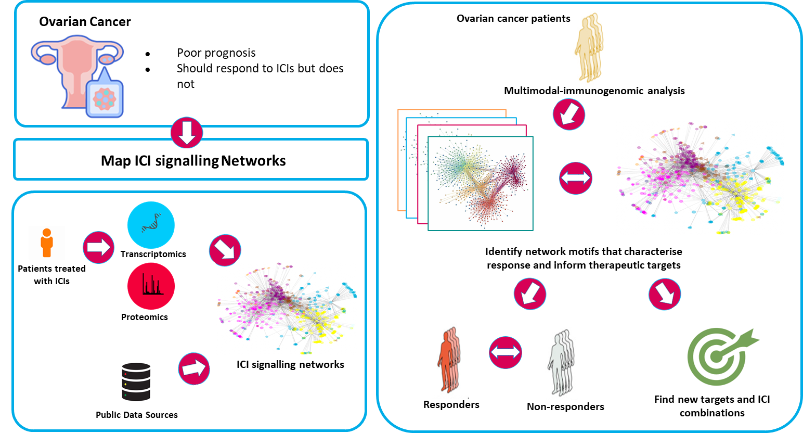

This project focuses on: i) Mapping the PD1 and CTLA4 signalling pathways by which ICIs mediate their effects; ii) Identifying new targets in ICI signalling pathways to improve ICIs and develop better combination therapies.
i) Little is known about PD1 and CTLA4 signalling. PD1 impinges on cell survival, cell cycle progression, metabolic pathways and TGFβ signalling, but the molecular mechanisms remain rather obscure. Using our experience in the reconstruction of biological signal transduction networks, we will map the signalling pathways used by PD1 and CTLA4 in T-cells deploying a combination of proteomics, transcriptomics and computational modelling.
ii) Here, we are applying computational models to analyse information processing by the ICI signal transduction pathways and identify ways to improve ICI efficacies. By evaluating the in silico prediction in various experimental models, we can make mechanism based recommendations for subsequent preclinical and clinical studies.
For further information, visit:
Project team



Dr. Luis Iglesias Martinez
SBI Investigator

Dr. Vadim Zhernovkov
Senior Investigator

Dr. Myriam Nabhan
Postdoctoral Researcher

Dr. Martina Kreileder
Postdoctoral Researcher


