

P2.2: microRNAs for the treatment of breast cancer
Plain Language Summary
Breast cancer impacts the lives of 2.2 million women each year globally with approximately 1 in 9 developing this disease in their lifetime in Ireland. Despite increased awareness, breast screening, and advancements in novel treatment options, there is still an urgent need for more targeted therapies particularly for those diagnosed with aggressive late-stage breast cancer. In this project, we are utilising the natural ability of extracellular vesicles (EVs) to carry microRNAs (miRNAs) to the site of injury where they will suppress the growth of the tumor.
In other words, EVs act like transport vehicles transporting parcels (miRNAs) to a house(tumour site).


This therapy has proven promising with a moderate reduction in the size of the tumour observed in previous studies by our group. The next step will be to see how the immune system responds to our therapy in a pre-clinical model of advanced breast cancer. This is important to determine any side effects before progression to clinical trials.
Visual Abstract
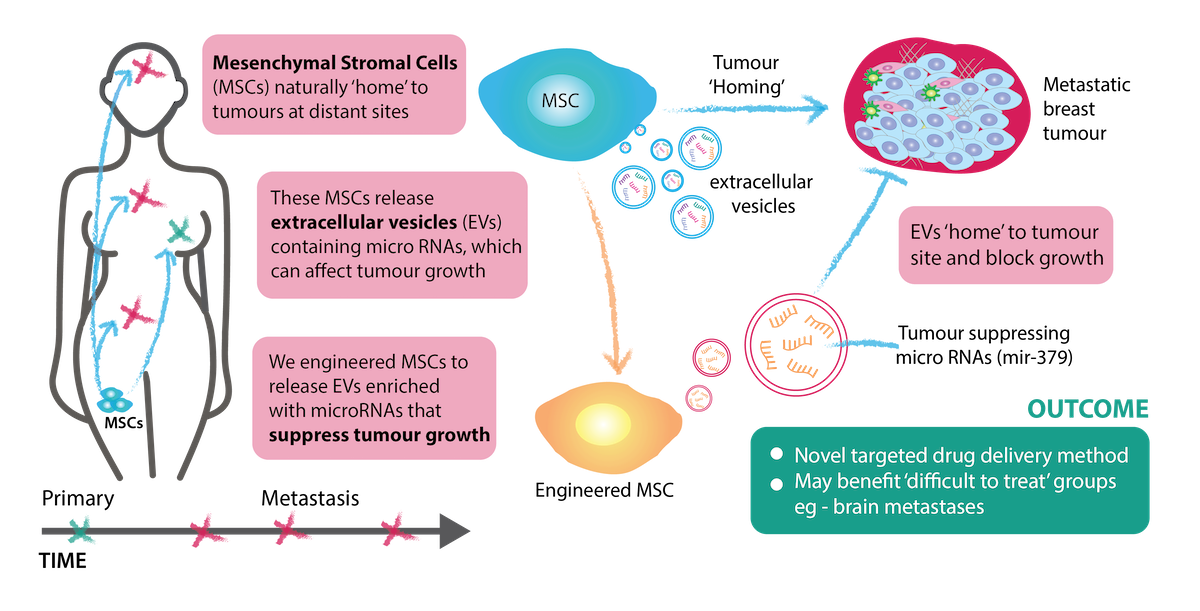
Project Overview
As our understanding of the crucial role of microRNAs (miRNAs) in cancer has developed, interest in tumour-targeted delivery of therapeutic miRNAs has increased. In this project, we are harnessing the natural tumour-tropism of extracellular vesicles (EVs) from Mesenchymal Stem Cells (MSCs) to deliver tumour suppressing miRNAs for treating advanced breast cancer that has spread to different sites.
MSCs can home to the sites of metastatic tumours while evading the host immune response. These characteristics have raised tremendous interest in their potential as tumour-targeted delivery vehicles for therapeutic agents, with the first clinical trial recently completed. MSCs release large quantities of EVs, which naturally contain miRNAs that can be transferred to recipient cells. We have previously shown that miR-379 is a potent tumour-suppressor that is significantly reduced in breast tumours compared to healthy tissue. To deliver miR-379, we engineered MSCs that secrete EVs enriched with miR-379 (EV-379) or a non-targeting control (EV-NTC). Administration of EV-379 was well tolerated and resulted in reduced tumour activity. As native MSC-EVs may retain the tumour-homing capability of MSCs, the next step will be to characterize EV-379 therapeutic potential in clinically relevant models of advanced disease with an intact immune system, which will allow us to study how EVs interact with the immune system and whether they will work well in combination with existing medicines.
This work uses both a new class of therapeutic and a new way to deliver it. In particular, the potential tumour homing properties and the ability of engineered EVs to efficiently cross the blood brain barrier would have major therapeutic benefits for treating metastatic breast cancer. Systemic treatment is very important for patients with brain metastases, and our EVs may critically expand treatment options for this group of patients, who would typically have very limited treatment options.
Key References:
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007 Jun;9(6):654-9. PubMed PMID: 17486113.
- Dwyer RM, Ryan J, Havelin RJ, Morris JC, Miller BW, Liu Z, et al. Mesenchymal Stem Cell (MSC) Mediated Delivery of the Sodium Iodide Symporter (NIS) Supports Radionuclide Imaging and Treatment of Breast Cancer. Stem Cells. 2011 May 23. PubMed PMID: 21608083.
- O'Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137-49. PubMed PMID: 29367765
- Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharmaceutical research. 2015 Jun;32(6):2003-14. PubMed PMID: 25609010. Pubmed Central PMCID: 4520542.
- Gilligan KE and Dwyer RM “Extracellular Vesicles (EVs) for Cancer Therapy: Impact of Host Immune Response” Cells 2020 Jan 16; 9(1) pii: E224; PMID: 31963216
For further information, visit:
(opens in a new window)https://research.universityofgalway.ie/en/persons/r%C3%B3is%C3%ADn-dwyer
Project team


Dr. Elan McCarthy
Postdoctoral Researcher
.jpg)
Dr. Lili Lin
Postdoctoral Researcher
.jpg)

